Boiling point of br2
Home » datasheet » Boiling point of br2Boiling point of br2
Boiling Point Of Br2. It is exothermic and there is an increase in disorder b. These two bases can form a connection between two strands of DNA. C R Br2 R-Br Br propagation step 2 Kharasch reports that free-radical substitution of cyclohexane with Br2 reacts very slowly in the dark or in the absence of oxygen. 557-91-5 - APQIUTYORBAGEZ-UHFFFAOYSA-N - 11-Dibromoethane - Similar structures search synonyms formulas resource links and other chemical information.
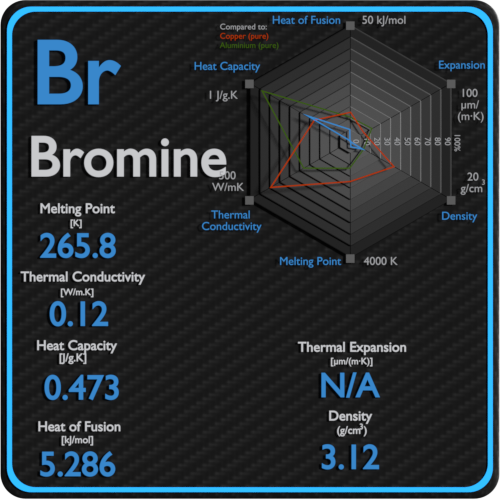 Bromine Thermal Properties Melting Point Thermal Conductivity Expansion From material-properties.org
Bromine Thermal Properties Melting Point Thermal Conductivity Expansion From material-properties.org
557-91-5 - APQIUTYORBAGEZ-UHFFFAOYSA-N - 11-Dibromoethane - Similar structures search synonyms formulas resource links and other chemical information. Oxidation number of free elements Free elements include molecular elements. A102 kJmol exothermic B102 kJmol endothermic C102 kJmol endothermic D102 kJmol exothermic hollloooowwweeennn. Except hydrogen in the hydride compounds compound of H with. H2 O2 O3 N2 F2 Cl2 Br2 I2 P4 S8 have oxidation number of 0 zero. Which one of the following substances is expected to have the lowest melting point.
HF NH3 H2O WHY.
Osmium is the densest naturally occurring element. Surface tension of water liquids and aqueous solutions table of values Dielectric constant of liquids gases and solids Table Dissociation constants of acids and bases. Double and single spacing. A102 kJmol exothermic B102 kJmol endothermic C102 kJmol endothermic D102 kJmol exothermic hollloooowwweeennn. Osmium from Greek ὀσμή osme smell is a chemical element with the symbol Os and atomic number 76. It is endothermic and there is an increase in disorder c.
 Source: youtube.com
Source: youtube.com
Double and single spacing. Oxidation number of free elements Free elements include molecular elements. In low concentration oxygen can act as a free-radical initiator forming Br radicals from Br2 but here Kharasch also observes that in high concentration oxygen can inhibit free-radical reactions. Oxidation number of hydrogen In its compounds oxidation number of H always 1. 12 point ArialTimes New Roman.
 Source: en.wikipedia.org
Source: en.wikipedia.org
2HBr H2 Br2 The energy of the reactant is 732 kJmol and the energy of the products is 630 kJmol. Except hydrogen in the hydride compounds compound of H with. Which one of the following substances is expected to have the highest boiling point. In low concentration oxygen can act as a free-radical initiator forming Br radicals from Br2 but here Kharasch also observes that in high concentration oxygen can inhibit free-radical reactions. Oxidation number of free elements Free elements include molecular elements.
 Source: material-properties.org
Source: material-properties.org
Which one of the following substances is expected to have the lowest melting point. Except hydrogen in the hydride compounds compound of H with. Sep 30 2021. H2 O2 O3 N2 F2 Cl2 Br2 I2 P4 S8 have oxidation number of 0 zero. Melting PointRange-72 C 19 F Boiling PointRange 587 C 1377 F Flash Point Not applicable Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper No data available Lower No data available Vapor Pressure 230 mbar 20 C Vapor Density 551 Air 10 Specific Gravity 3111 Solubility No information available.
 Source: clutchprep.com
Source: clutchprep.com
C R Br2 R-Br Br propagation step 2 Kharasch reports that free-radical substitution of cyclohexane with Br2 reacts very slowly in the dark or in the absence of oxygen. Which one of the following substances is expected to have the highest boiling point. Which of the following statements best helps explain this observation. All our clients are privileged to have all their academic papers written. C R Br2 R-Br Br propagation step 2 Kharasch reports that free-radical substitution of cyclohexane with Br2 reacts very slowly in the dark or in the absence of oxygen.
 Source: toppr.com
Source: toppr.com
Which one of the following substances is expected to have the highest boiling point. What is the total energy of the reaction. Which one of the following substances is expected to have the lowest melting point. 2HBr H2 Br2 The energy of the reactant is 732 kJmol and the energy of the products is 630 kJmol. What advantages do you get from our Achiever Papers services.
 Source: youtube.com
Source: youtube.com
In low concentration oxygen can act as a free-radical initiator forming Br radicals from Br2 but here Kharasch also observes that in high concentration oxygen can inhibit free-radical reactions. Which one of the following substances is expected to have the highest boiling point. A102 kJmol exothermic B102 kJmol endothermic C102 kJmol endothermic D102 kJmol exothermic hollloooowwweeennn. 557-91-5 - APQIUTYORBAGEZ-UHFFFAOYSA-N - 11-Dibromoethane - Similar structures search synonyms formulas resource links and other chemical information. In low concentration oxygen can act as a free-radical initiator forming Br radicals from Br2 but here Kharasch also observes that in high concentration oxygen can inhibit free-radical reactions.
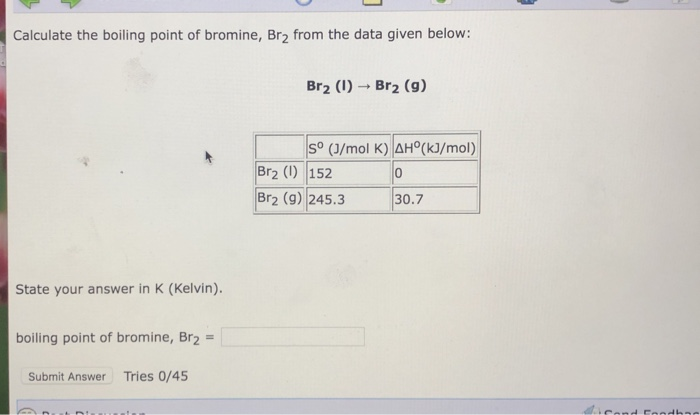 Source: chegg.com
Source: chegg.com
Melting PointRange-72 C 19 F Boiling PointRange 587 C 1377 F Flash Point Not applicable Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper No data available Lower No data available Vapor Pressure 230 mbar 20 C Vapor Density 551 Air 10 Specific Gravity 3111 Solubility No information available. Double and single spacing. Is this reaction endothermic or exothermic. The carbon chains are longer in nonane than they are in 234-trifluoropentane. Oxidation number of free elements Free elements include molecular elements.

When experimentally measured using x-ray crystallography it has a density of 2259 gcm 3. Osmium is the densest naturally occurring element. We are here to help you if you are stuck not to do your work. HF NH3 H2O WHY. H2 O2 O3 N2 F2 Cl2 Br2 I2 P4 S8 have oxidation number of 0 zero.
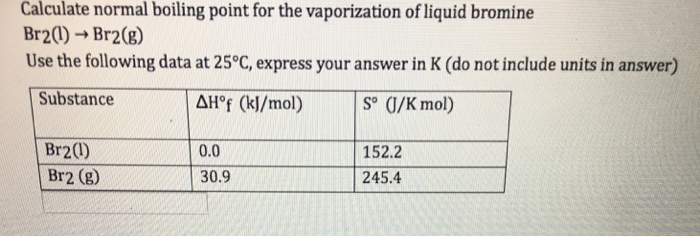
It is endothermic and there is an increase in disorder c. All our academic papers are written from scratch. All our clients are privileged to have all their academic papers written. Melting PointRange-72 C 19 F Boiling PointRange 587 C 1377 F Flash Point Not applicable Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper No data available Lower No data available Vapor Pressure 230 mbar 20 C Vapor Density 551 Air 10 Specific Gravity 3111 Solubility No information available. HF NH3 H2O WHY.
 Source: chegg.com
Source: chegg.com
C R Br2 R-Br Br propagation step 2 Kharasch reports that free-radical substitution of cyclohexane with Br2 reacts very slowly in the dark or in the absence of oxygen. The carbon chains are longer in nonane than they are in 234-trifluoropentane. H2 O2 O3 N2 F2 Cl2 Br2 I2 P4 S8 have oxidation number of 0 zero. Estimate the boiling point of water if for H2O 2858 kJmol and S0 6991 JmolK and for H2Og 2418 kJmol and S0 1887 JmolK. Oxidation number of fluorine In its compounds oxidation number of F always 1.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of br2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.