Boiling point of cyclohexane
Home » datasheet » Boiling point of cyclohexaneBoiling point of cyclohexane
Boiling Point Of Cyclohexane. Alkanes consist of four carbon atoms at standard temperatures. The boiling point constant for cyclohexane is 279 Cm. To achieve the goal of producing a provisional or preliminary physical property model measurements of chemical composition thermal decomposition density viscosity thermal conductivity. The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent.
 Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
147 g 16214 gmol 0647 kg 140127 m. Δt i K b m. Freezing point of the fluid 79 C has made this fluid the only air- breathing missile fuel used by the United States at the present time. Highly branched vs. DMSO nitrobenzene octanoles sulfuric acid. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester.
Research Triangle Park North Carolina.
For example if we synthesized a known liquid that boiled at 120-122 C this value could be used to confirm that we prepared what we were interested in and that our substance was reasonably pure. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. High molecular or high boiling compounds. Consider for example ethanol consisting of a weight concentration of approximately ninety-five per cent and four per cent of water. Dioxane methanol ethanol nitric acid nitromethane pyridine phosphorous oxychloride. Bromine chloroform cyclohexane ethyl acetate triethylamine.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Soluble in non-polar solvents such as benzene and cyclohexane. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester. Stability and Reactivity Stability. According to this CI scale all n-paraffins have a CI value of 0 while cyclohexane the simplest naphthene has a CI value of 50 and benzene has a CI value of 100. The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
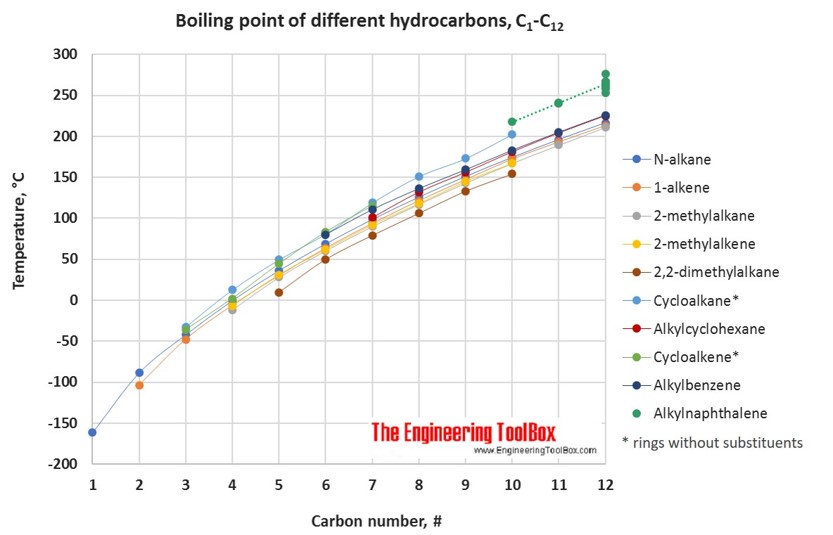
As a final example I give you 2233-tetramethylbutane. 4 Spectral Information Expand this section. Stable at room temperature in sealed containers. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Highly branched vs.
 Source: chegg.com
Source: chegg.com
ILO International Chemical Safety Cards ICSC-050 C. Maximum Boiling Azeotropes or Positive Azeotrope. 2 We utilize this formula. As a final example I give you 2233-tetramethylbutane. The boiling and melting point of alkane depends upon the length of the carbon chain.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
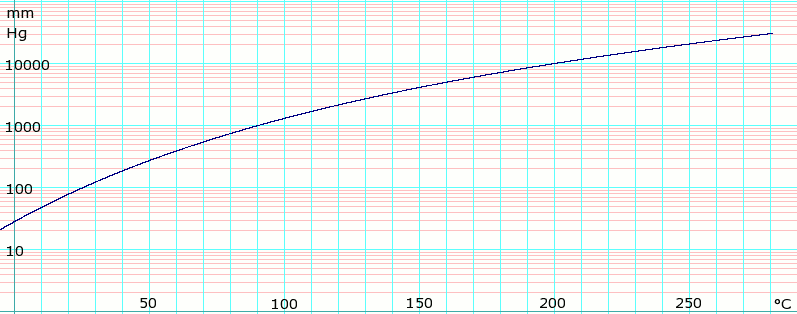
Carbon dioxide and carbon monoxide may form when heated to. Solvent formula polarity boiling point 0C water H2O very polar 100 ethanol CH3CH2OH polar 78 methanol CH3OH polar 65. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Using the CI values. Binary mixture VLE data at a certain overall pressure such as 1 atm showing mole fraction vapor and liquid concentrations when boiling at various temperatures can be shown as a two-dimensional graph called a boiling-point diagram.

2 Names and Identifiers Expand this section. Dioxane methanol ethanol nitric acid nitromethane pyridine phosphorous oxychloride. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. This can be exemplified by looking at the BP of water at different pressures. Water boils at 373 K and ethanol boils at.
 Source: en.wikipedia.org
Source: en.wikipedia.org
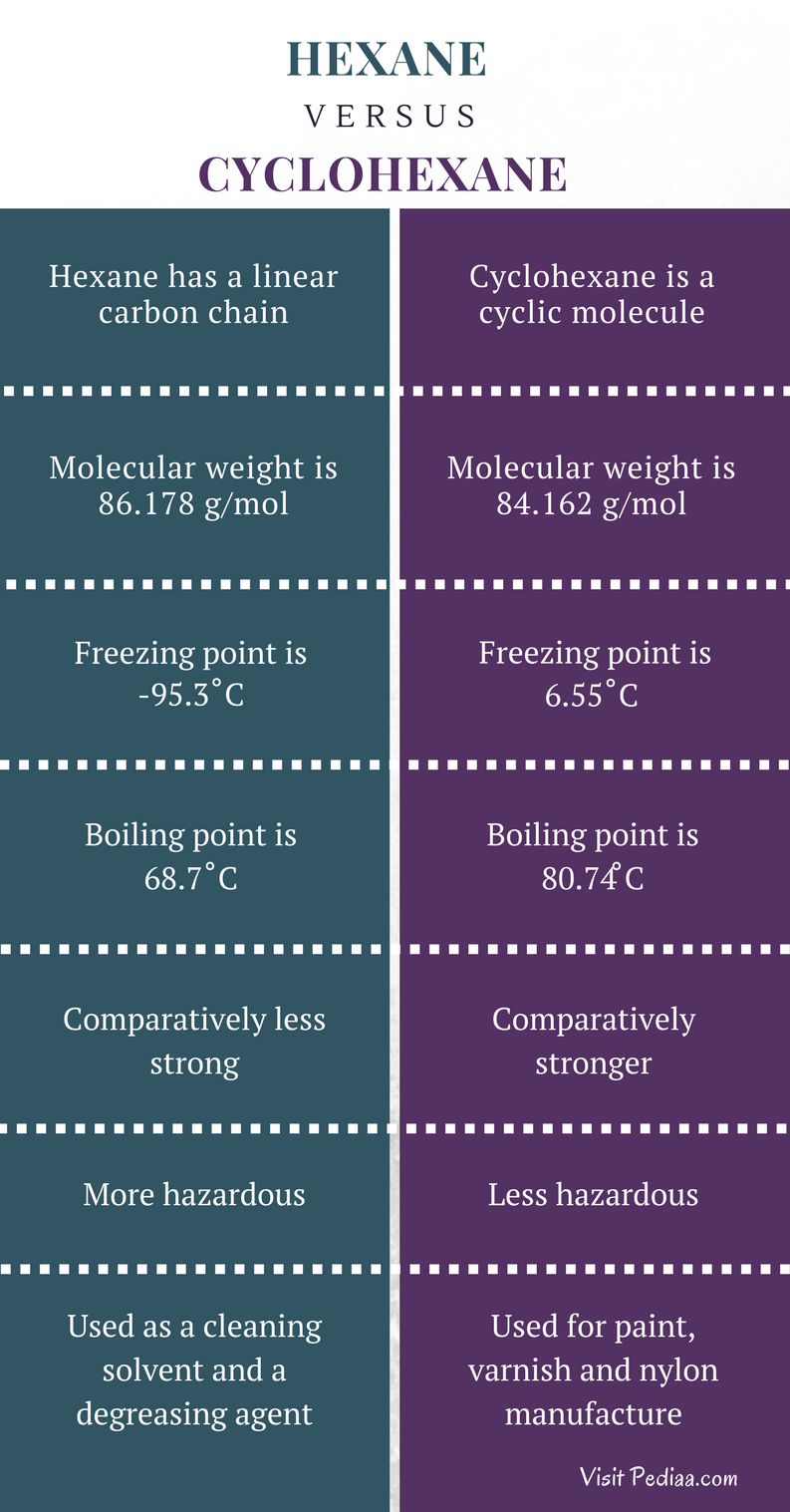
If a single solvent cannot be found that is suitable for recrystallization a solvent pair often used. ILO International Chemical Safety Cards ICSC-050 C. 3 Chemical and Physical Properties Expand this section. Separation of cyclohexane and benzene. It has a role as a human xenobiotic metabolite.

1 Determine the molality of the lactic acid solution. The boiling point constant for cyclohexane is 279 Cm. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. 95 20C 68F Evaporation Rate BuAc1. Stable at room temperature in sealed containers.

Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. Its not a straightforward topic. Some commonly used solvent pairs are water-ethanol acetic. Stable at room temperature in sealed containers. Solvent formula polarity boiling point 0C water H2O very polar 100 ethanol CH3CH2OH polar 78 methanol CH3OH polar 65.
 Source: pediaa.com
Source: pediaa.com
Consider for example ethanol consisting of a weight concentration of approximately ninety-five per cent and four per cent of water. Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model. Water butanoles propanoles aniline toluene bromoform dimethylformamide. 2 We utilize this formula. The majority of solvent cleaning work is performed in equipment of two types relative to flash point either an open tank or a closed tank.

Alkanes comprising of more than three carbon atoms are capable of. 2 We utilize this formula. 1 Structures Expand this section. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. The mole fraction of component 1 in the mixture can be represented by the symbol x 1.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of cyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.