Znso4 boiling point
Home » datasheet » Znso4 boiling pointZnso4 boiling point
Znso4 Boiling Point. Chemical properties of metals and non metals. High melting point high boiling point high density hard good conductor of electricity good conductor of heat malleable and ductile useful catalyst compounds are coloured anything else which seems sensible a Ne atoms have 8 electrons in their outer level. Side effects of excess supplementation may include abdominal pain vomiting headache and tiredness. A Trigonal planar b Tetrahedral c T-Shaped d Distorted.
 Thermodynamic Properties Of Znso4 Aq And Phase Equilibria In The Znso4 H2o System From 268 K To 373 K Sciencedirect From sciencedirect.com
Thermodynamic Properties Of Znso4 Aq And Phase Equilibria In The Znso4 H2o System From 268 K To 373 K Sciencedirect From sciencedirect.com
Metal oxygen metal oxide. 1 Reaction With Air. Fill in the total number of atoms present for each type of molecule. Matter occupies space and has mass. I Effect of pressure The boiling point of a liquid increases with increase in external pressure. Question 2 Using the following information do the conversions shown below showing all work.
Shape of ClF3 is.
Chemical properties of metals and non metals. A Low dissociation energy of F2 molecule b Associated nature due to hydrogen bonding c Ionic character of HF d High electronegativity of fluorine 15. The high viscosity and high boiling point of HF is due to. Note The melting and freezing points of any pure substance have the same value. Oxygen is prepared in the lab by catalytic decomposition of hydrogen peroxide using manganese IV oxideas shown. Oxygen may be obtained from the atmosphere by the liquefaction and fractional distillation of air.
Source:
A Low dissociation energy of F2 molecule b Associated nature due to hydrogen bonding c Ionic character of HF d High electronegativity of fluorine 15. White vitriol zinc sulphate heptahydrate zincvitriol heptahydrate 12. 1 ft 12 inches 1 pound 16 oz 1 gallon 4 quarts 1 mile 5280 feet 1 ton 2000 pounds 1. Heat of Condensation B. Chemical properties of metals and non metals.
Source:
215 g Molar mass of AgNO3 is 169. 1 The states of matter 5 Revision questions 1 Give the FOUR main ideas behind the particulate theory of matter. II and III E. Ne Rydberg Calculator is package of routines written in. Mg O2 MgO.
 Source: researchgate.net
Source: researchgate.net
Zn H 2SO 4 gives dil ZnSO4 H20 Above reaction is - a decomposition reaction b single displacement reaction c combination reaction d synthesis reaction 240. Free essays homework help flashcards research papers book reports term papers history science politics. The most powerful oxidising agent is. No room to share electrons to form covalent bonds and therefore monatomic. The freezing point is the constant temperature at which a liquid changes state into a solid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which has most stable 2 oxidation state. This is the reason why water boils at a lower temperature than 100C at higher altitudes. Boiling point of hydrocarbons simple alcohols and carboxylic acids increase with an increase in molecular weight A. I II and III. NH4NO3 mc018-1jpg N2O 2H2O A chemist who is performing this reaction starts with 1601 g of NH4NO3.
 Source: sciencedirect.com
Source: sciencedirect.com
BRILLIANT PUBLIC SCHOOL SITAMARHI Affiliated up to 2 level to CBSE New Delhi Class-XI IIT-JEE Advanced Chemistry Study Package Session. Solids liquids and gases. Convert 10053 to exponential form and explain your answer. The electron is a subatomic particle symbol e or β with a. Factors Affecting the Boiling Point Though every liquid is characterized by specific value for the boiling point it depends on some factors as discussed hereunder.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A Low dissociation energy of F2 molecule b Associated nature due to hydrogen bonding c Ionic character of HF d High electronegativity of fluorine 15. Relevant identified uses of the substance or mixture and uses advised against Use of the substancemixture. The boiling point is the constant temperature at which a liquid changes state into a gas. I and II D. Zn H 2SO 4 gives dil ZnSO4 H20 Above reaction is - a decomposition reaction b single displacement reaction c combination reaction d synthesis reaction 240.
 Source: textilesgreen.in
Source: textilesgreen.in
The gas is then collected over water. —– ABSTRACT This manual provides test procedures approved for the monitoring of water supplies waste discharges and ambient waters under the Safe Drinking Water Act the National Pollutant Discharge Elimination System and Ambient Monitoring Requirements of Section 106 and 208 of Public Law 92-500 The test methods have been selected to meet the needs of federal legislation and to. A MgCl2 Mg 1 B 5 ZnSO4 Zn 5 Dec 17 2010 CDK Descriptor Calculator GUI v1. Heat of Fusion C. Calculate the percentage of M n O 2 in the sample.
Source: chemspider.com
PON PURE CHEMICALS GROUP CHENNAI TAMILNADU INDIA 24 Hour Health Emergency 91 8939878447 91 9444038694 Transportation Emergency Phone 91 9444038517 Company Name Place EMERGENCY TELEPHONE NUMBER PON PURE CHEMICALS GROUP India Day Emergency 044 -26161803 -26161809 2. This is the reason why water boils at a lower temperature than 100C at higher altitudes. The molar mass of water H2O is 1801 gmol. Side effects of excess supplementation may include abdominal pain vomiting headache and tiredness. 1995S a Sn b Pb c Fe d Ag 39.
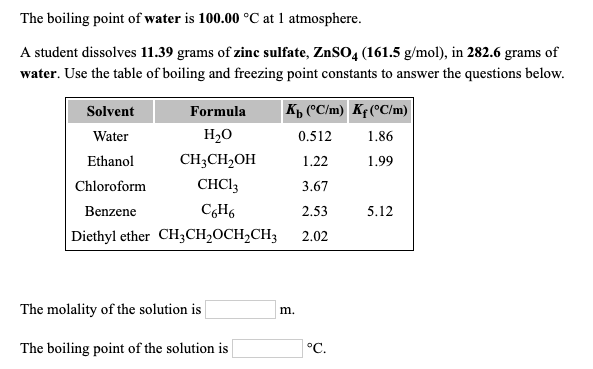 Source: chegg.com
Source: chegg.com
Convert the melting point of the metal obtained from copperI sulfide ore to degrees Celsius. I II and III. Zn H 2SO 4 gives dil ZnSO4 H20 Above reaction is - a decomposition reaction b single displacement reaction c combination reaction d synthesis reaction 240. Amongst H2O H2S H2Se and H2Te the one with the highest boiling point is 2000S a H2O because of hydrogen bonding b H2Te because of higher molecular weight c H2S because of hydrogen bonding d H2Se because of lower molecular weight 38. The high viscosity and high boiling point of HF is due to.
 Source: coolgyan.org
Source: coolgyan.org
I and II D. Heat of Vaporization D. The most powerful oxidising agent is. Boiling point of hydrocarbons simple alcohols and carboxylic acids increase with an increase in molecular weight A. The high viscosity and high boiling point of HF is due to.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title znso4 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.