Zncl2 melting point
Home » datasheet » Zncl2 melting pointZncl2 melting point
Zncl2 Melting Point. ZINC chloride ZnCl2 or Cl2Zn CID 5727 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Volume πr2h 314163 x 10 4 cm22 x 10 4 cm 6 x 10 11 cm3 Ballpark estimate. You may wish to assign various lab. Ideal Gas Heat of Formation -13318X108 Jkmol.
 Pressure Temperature Phase Diagram Of Zncl 2 Including Solid Lines And Download Scientific Diagram From researchgate.net
Pressure Temperature Phase Diagram Of Zncl 2 Including Solid Lines And Download Scientific Diagram From researchgate.net
The lowest first ionization energies in a period. Ideal Gas Heat of Formation -13318X108 Jkmol. Inform them that this is the melting point of ice and that the freezing point of water equals the melting point of ice. The melting point of gold. Have students then add 10 grams of antifreeze to the 90 grams of ice in the cup and measure the freezing point of the 10 antifreeze solution by recording the lowest temperature reached. Hazardous Substances Data Bank HSDB 326 Flash Point.
The lowest first ionization energies in a period.
Academiaedu is a platform for academics to share research papers. It has a specific heat of 060 in its liquid state. As indicated by Fig. 0 F USCG 1999 US. This white salt is hygroscopic and even deliquescentSamples should therefore be protected from sources of moisture including the water vapor present in ambient air. A there is a very large difference in the ionic character of the Al-F and Si - F bonds Bin AIFS.
Melting point of this alloy 945C Melting point of Pb 327C Melting point of Sn 2319C Melting point of Bi 271C Freezing. Record the freezing point in their data chart. This is carried out by extracting heat from the liquid. What is an electrochemical cell. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
 Source: byjus.com
Source: byjus.com
This is carried out by extracting heat from the liquid. Very high melting point e. Ideal Gas Heat of Formation -13318X108 Jkmol. Melting point of this alloy 945C Melting point of Pb 327C Melting point of Sn 2319C Melting point of Bi 271C Freezing. And para-isomer has the higher boiling point than ortho-isomer.
 Source: researchgate.net
Source: researchgate.net
The addition of NaCl in the presence of HCl slightly increases the solubility of CuCl 2 but the presence of FeCl 3 andor ZnCl2 affects its solubility to a greater extent especially the presence of FeCl 3. You may wish to assign various lab. Therefore they can be easily separated from each other. 5 x 10S11 cm3 S 117 S S S 1 carat 200 mg 200 x 10S3 g 0200 g Mass of Hope Diamond in grams 444. Ideal Gas Heat of Formation -13318X108 Jkmol.
Source: chemspider.com
The melting points of both the isomer differ significantly. Liquid Molar Volume 0088777 cu mkmol. The addition of NaCl in the presence of HCl slightly increases the solubility of CuCl 2 but the presence of FeCl 3 andor ZnCl2 affects its solubility to a greater extent especially the presence of FeCl 3. The symbol M represents any one of the alkaline earth metals a. Melting point increases by 100.
 Source: en.wikipedia.org
Source: en.wikipedia.org
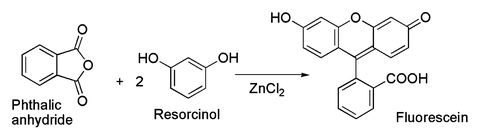
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Volume πr2h 314163 x 10 4 cm22 x 10 4 cm 6 x 10 11 cm3 Ballpark estimate. Zinc Chloride Preparation The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride. When a substance in the liquid state is subjected to cooling heat is. 33 x 10S4 cm22 x 10S4 cm.
 Source: softschools.com
Source: softschools.com
O Fb r d2 3 x 10 6 m 3 x 10 4 cm. The lowest first ionization energies in a period. The symbol M represents any one of the alkaline earth metals a. Therefore they can be easily separated from each other. Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Therefore they can be easily separated from each other. Very high melting point e. Inform them that this is the melting point of ice and that the freezing point of water equals the melting point of ice. The latent heat of fusion of alcohol is 50 kcalkg and its melting point is -54circC. Most of the elements on the periodic table are ____.
 Source: researchgate.net
Source: researchgate.net
Which of the following halogens would have the highest melting point. Preparation of aryl chloride and bromide is possible from this reaction. Most of the elements on the periodic table are ____. Thus the reaction forms two different isomers of the product-ortho and para. Taylor and Francis 1989.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Very high melting point e. Have students then add 10 grams of antifreeze to the 90 grams of ice in the cup and measure the freezing point of the 10 antifreeze solution by recording the lowest temperature reached. Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates. Melting point of this alloy 945C Melting point of Pb 327C Melting point of Sn 2319C Melting point of Bi 271C Freezing. And para-isomer has the higher boiling point than ortho-isomer.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The effect of a variety of metal-chlorides additions on the melting behavior and thermal stability of commercially available salts was investigated. And para-isomer has the higher boiling point than ortho-isomer. H 2 x 10 6 m 2 x 10 4 cm S S S Calculation. Table- Atomic And Physical Properties Of Isotopes Hydrogen Property Hydrogen Deuterium Tritium Active Abundance 99985 00156 -15 10 Relative at mass 1008 2014 3016 Melting point 1396 1873 2062 Boiling point 2039 2367 250 Density 009 018 027 E of fusion 0117 0197 _ E of vaporization 0904 0197 _ E of dissociation 43588 44335 _ Interneuclar dist 7414 7414 _ Electronic. Liquid Molar Volume 0088777 cu mkmol.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zncl2 melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.