Zinc chloride hydrogen chloride boiling point
Home » datasheet » Zinc chloride hydrogen chloride boiling pointZinc chloride hydrogen chloride boiling point
Zinc Chloride Hydrogen Chloride Boiling Point. The chemical equation for this reaction is given by. H 2 S and H 2 O are. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. These rapidly turn back to the solid forming a fine powder.
 Zinc Chloride Wikipedia From en.wikipedia.org
Zinc Chloride Wikipedia From en.wikipedia.org
Ammonium chloride increases acidity by increasing the amount of hydrogen ion concentrations. Why H 2 S having less boiling point than H 2 O H 2 S boiling point. But water H 2 O is a liquid. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Ullmanns Encyclopedia of Industrial Chemistry. Federal Republic of Germany.
Ammonium chloride can be used as an expectorant due to its irritative action on the bronchial mucosa.
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. At room temperature hydrogen disulfide H 2 S is a gas. The ICSC project is a common undertaking between the World Health Organization WHO and. Melting point - the temperature at which a solid turns into a liquid. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: en.wikipedia.org
Source: en.wikipedia.org
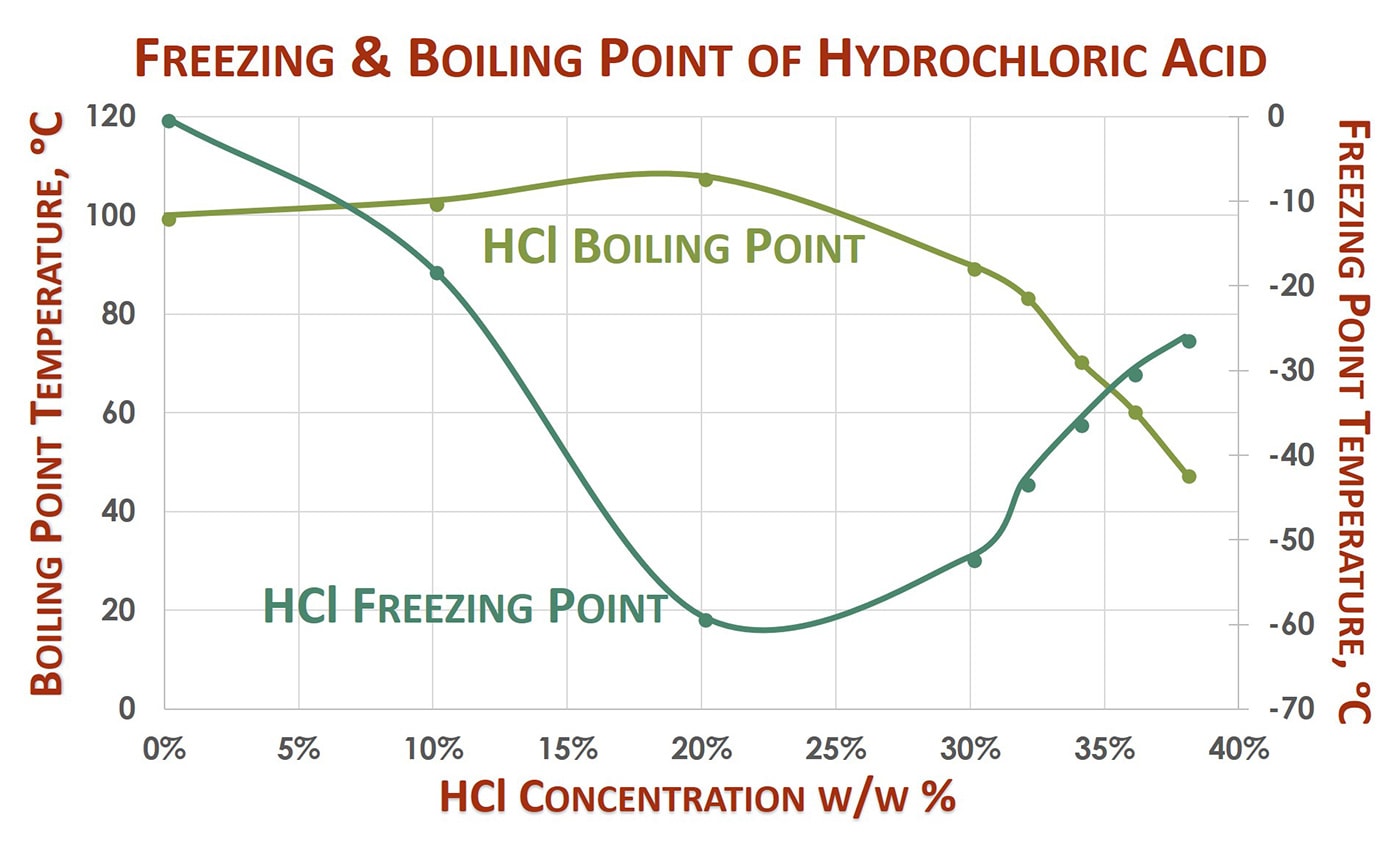
2003 to Present p. Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates. Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. The boiling point of zinc chloride is about 730 C. Boiling point - the temperature at which a liquid turns into a gas.
 Source: youtube.com
Source: youtube.com
3646 EPA 1998 Water Solubility. This white salt is hygroscopic and even deliquescentSamples should therefore be protected from sources of moisture including the water vapor present in ambient air. Ullmanns Encyclopedia of Industrial Chemistry. The boiling point of zinc chloride is about 730 C. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
3646 EPA 1998 Water Solubility. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. If the zinc chloride does begin to boil it can boil over from the crucible and will also produce fumes of zinc chloride in the air. This can easily be reached by the combination of the heat from the Bunsen burner and the electric current. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans.

Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. This can easily be reached by the combination of the heat from the Bunsen burner and the electric current. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Melting point - the temperature at which a solid turns into a liquid.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. Ullmanns Encyclopedia of Industrial Chemistry. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans. Ammonium chloride increases acidity by increasing the amount of hydrogen ion concentrations. Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. MercuryII chloride or mercuric chloride historically also known as corrosive sublimate is the chemical compound of mercury and chlorine with the formula HgCl 2It is white crystalline solid and is a laboratory reagent and a molecular compound that is very toxic to humans. Ullmanns Encyclopedia of Industrial Chemistry. Hydrochloric acid also reacts with zinc sulphide to form zinc chloride and hydrogen. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Melting point - the temperature at which a solid turns into a liquid. Hydrochloric acid also reacts with zinc sulphide to form zinc chloride and hydrogen. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health.
 Source: protank.com
Source: protank.com
It is possible to confuse the boiling. Boiling point - the temperature at which a liquid turns into a gas. The boiling point of zinc chloride is about 730 C. Melting point - the temperature at which a solid turns into a liquid. It is possible to confuse the boiling.
 Source: byjus.com
Source: byjus.com
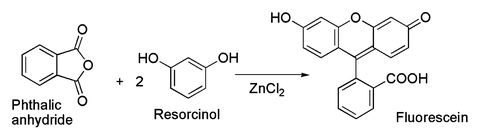
The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride. This effect causes the production of respiratory tract fluid which in order facilitates the effective cough. Zinc chloride is the name of chemical compounds with the formula ZnCl 2 and its hydrates. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. These rapidly turn back to the solid forming a fine powder.
 Source: en.wikipedia.org
Source: en.wikipedia.org
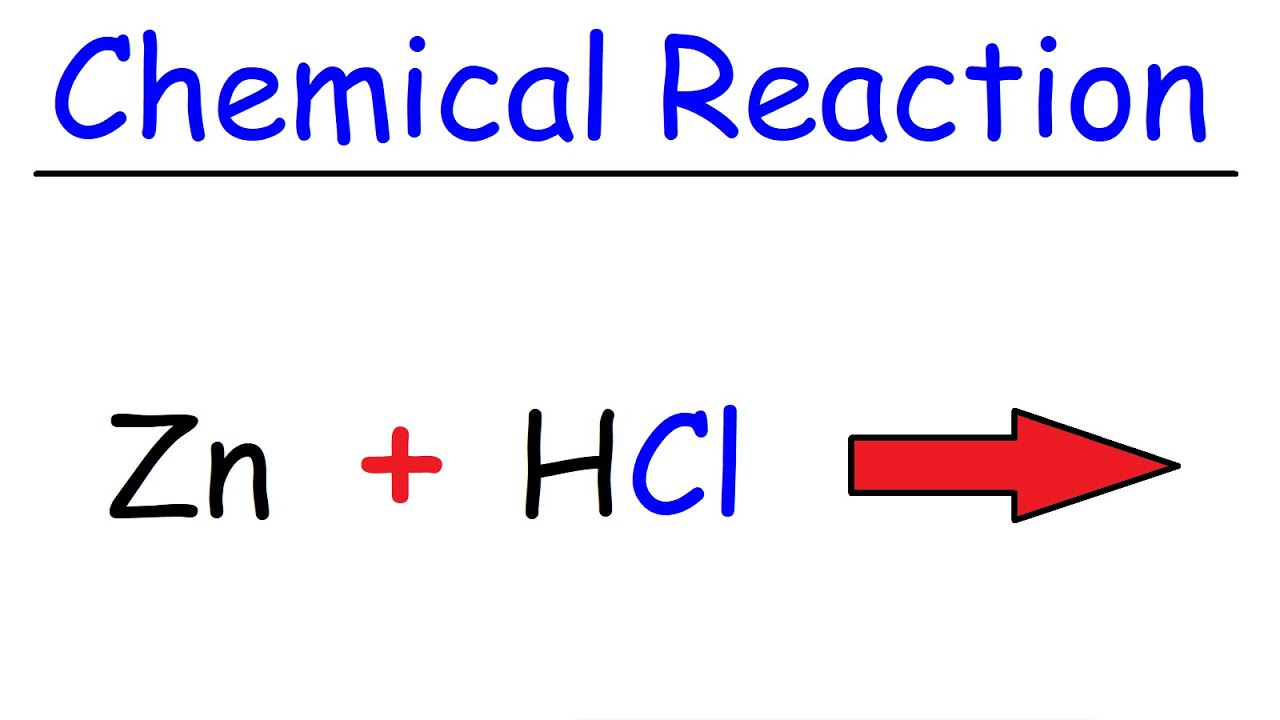
The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride. If the zinc chloride does begin to boil it can boil over from the crucible and will also produce fumes of zinc chloride in the air. Federal Republic of Germany. Zn 2HCl ZnCl 2 H 2. Ammonium chloride can be used as an expectorant due to its irritative action on the bronchial mucosa.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zinc chloride hydrogen chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.