Zinc chloride boiling point
Home » datasheet » Zinc chloride boiling pointZinc chloride boiling point
Zinc Chloride Boiling Point. This compound reacts with ammonia to form complexes. If you use a. 011 nm 2 Isotopes or mercury. It is brittle.
Zinc Chloride Cl2zn Chemspider From chemspider.com
Standard potential - 0763 V. Energy of first ionisation. Chair-Elect 2007 Chair 2008 Immediate Past Chair 2009 American Chemical Society Division of Chemical Education. The chief zinc mineral is the sulfide sphalerite zinc blende which together with its oxidation. The process involves the formation of cross-links between long rubber molecules so as to achieve improved elasticity resilience tensile strength viscosity hardness and weather resistance. However other compounds are present in table salt depending on its source or additives that may be included before packaging.
10 Reasons to Play Golf.
Energy of first ionisation. Air Liquide has gathered data on the compatibility of gases with materials to assist you in evaluating which materials to use for a gas system. Standard potential - 0763 V. Boiling point - the temperature at which a liquid turns into a gas. The process involves the formation of cross-links between long rubber molecules so as to achieve improved elasticity resilience tensile strength viscosity hardness and weather resistance. Ionic radius of mercury.
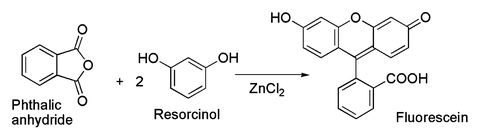 Source: en.wikipedia.org
Source: en.wikipedia.org
Zinc chlorides of which nine crystalline forms are known are colorless or white and are highly soluble in water. 10 Reasons to Play Golf. Molten zinc chloride is highly viscous and has a relatively low electrical conductivity value. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. Properties uses and occurrence.
 Source: en.wikipedia.org
Source: en.wikipedia.org
D Density g cm 3. Zinc is an element with atomic symbol Zn atomic number 30 and atomic weight 6539. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. Because of its comparative scarcity brilliant white colour malleability ductility and resistance to atmospheric. Golf has been played for at least 400 years according to the Spirit of Golf Foundation It began in Scotland spread to England and has since extended its reach around the globe.
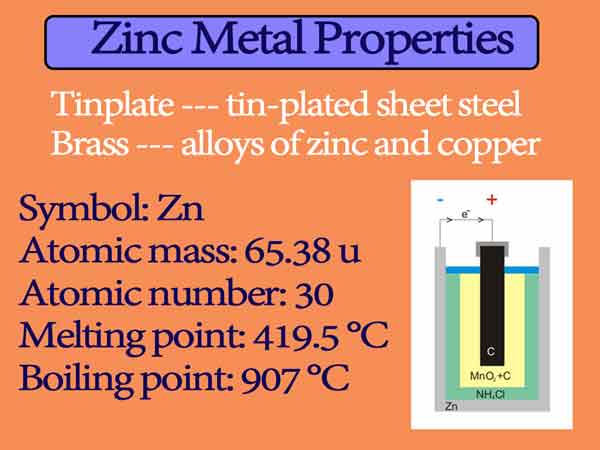 Source: chemistrypage.in
Source: chemistrypage.in
It is possible to confuse the boiling. Ullmanns Encyclopedia of Industrial Chemistry. Standard potential of mercury 0854 V Hg2 Hg Density of Mercury. This white salt is hygroscopic and even deliquescentSamples should therefore be protected from sources of moisture including the water vapor present in ambient air. Melting point - the temperature at which a solid turns into a liquid.
 Source: sciencedirect.com
Source: sciencedirect.com
Regularly clean your faucets screen also known as an aerator. Copper Tubes - Corrosion. Wiley-VCH Verlag GmbH Co. Electronic shell Ar 3d 10 4s 2. Standard potential - 0763 V.
Source: chemspider.com
Ionic radius of mercury. If lead particles are caught in the aerator lead can get into your water. NCI Thesaurus NCIt Contents. Chair Biennial Conference Committee Division of Chemical Education American Chemical Society 2014current. Energy of first ionisation.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Together with gold and the platinum-group metals silver is one of the so-called precious metals. Electron configuration Ar3d 10 4s 2. Zinc compounds are widely used in industry to make paint rubber dyes wood preservatives and ointments. Co-Chair ACS Examinations Institute 2015 General Chemistry Conceptual Exam Committee. Chair Biennial Conference Committee Division of Chemical Education American Chemical Society 2014current.
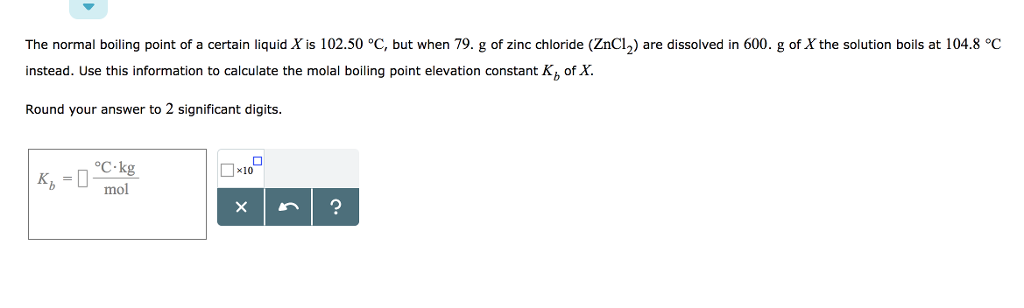 Source: chegg.com
Source: chegg.com
Therefore zinc does not contribute much electrons to the metallic lattice like other 3d metals. Electron configuration Kr4d 10 5s 1. This compound reacts with ammonia to form complexes. Occurrence uses and properties. Therefore zinc does not contribute much electrons to the metallic lattice like other 3d metals.
 Source: byjus.com
Source: byjus.com
Chair-Elect 2007 Chair 2008 Immediate Past Chair 2009 American Chemical Society Division of Chemical Education. Vanadium has the highest melting point and zinc has the lowest melting point. Energy of first ionisation. Co-Chair ACS Examinations Institute 2015 General Chemistry Conceptual Exam Committee. Sediment debris and lead particles can collect in your aerator.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Zinc is a lustrous bluish-white metal. Van Der Waals radius. Boiling point - the temperature at which a liquid turns into a gas. Vanadium has the highest melting point and zinc has the lowest melting point. Together with gold and the platinum-group metals silver is one of the so-called precious metals.
Because of its comparative scarcity brilliant white colour malleability ductility and resistance to atmospheric. Table salt is one of the most common household chemicals. NCI Thesaurus NCIt Contents. Why zinc has the lowest melting point in 3d metal series. If the zinc chloride does begin to boil it can boil over from the crucible and will also produce fumes of zinc chloride in the air.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zinc chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.