Why does benzoic acid have a high melting point
Home » datasheet » Why does benzoic acid have a high melting pointWhy does benzoic acid have a high melting point
Why Does Benzoic Acid Have A High Melting Point. In the second trial the temperature is increased rapidly to 370 ºC and then the rate of heating is decreased. Calculate the percent recovered using the following written formula and determine the melting point of your recrystallized benzoic acid. Another melting point trial of the same solid is then carried out. Salts and esters of butyric acid are known as butyrates or butanoates.
 Solved Why Does Benzoic Acid Have A Higher Melting Point Than Stearic Acid From numerade.com
Solved Why Does Benzoic Acid Have A Higher Melting Point Than Stearic Acid From numerade.com
Scoop a small amount of. Green the name of the person submitting the sample 11596 date submitted DLG 12-14 which is a reference to notebook pages concerning this material. Benzoic acid 140 g is reacted with oxygen in a constant volume calorimeter to form H2Ol and CO2g at 298 K. Improve this answer. In the second trial the temperature is increased rapidly to 370 ºC and then the rate of heating is decreased. More complex organic acids also have a variety of different functions.
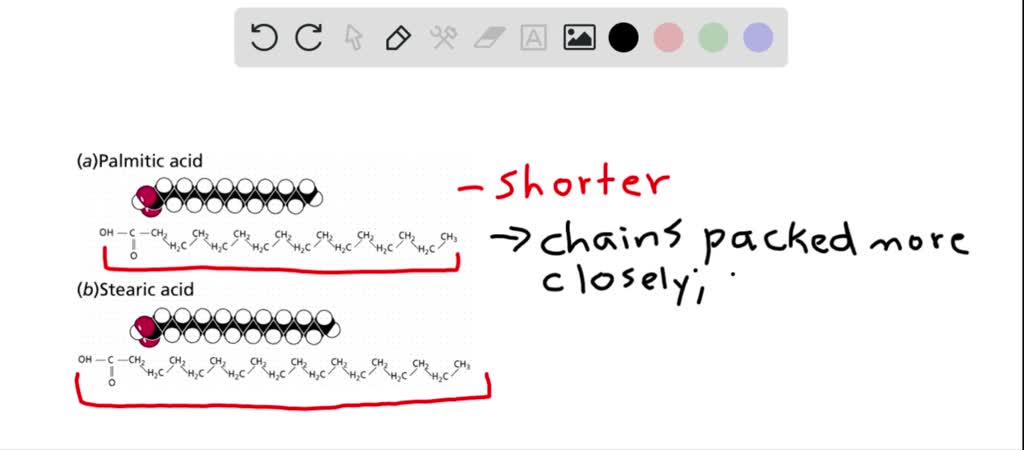
For example symmetrical neopentane molecules have a higher melting point than isopentane in which molecules do not pack well.
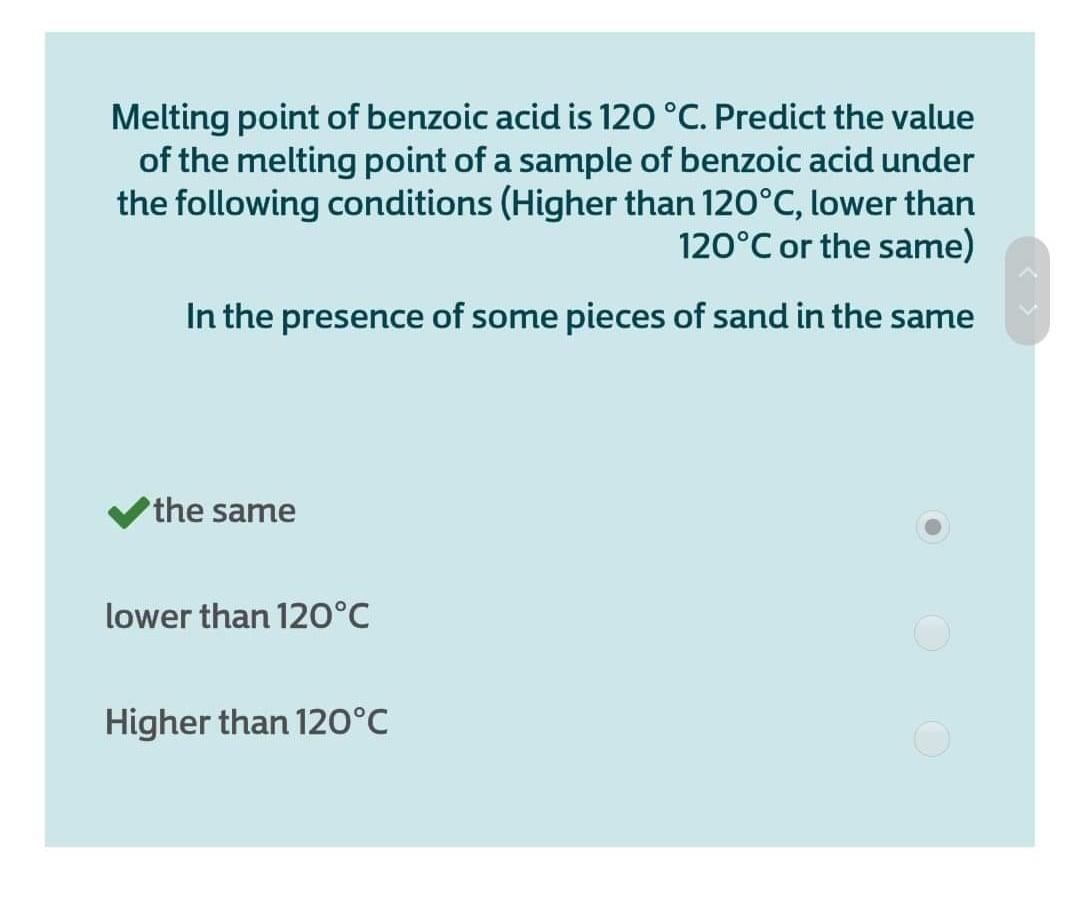
626k 11 11 gold badges 163 163 silver badges 347 347 bronze badges endgroup Add a comment 0 begingroup If i is the impure aspirin sample then yes. Ask the students to describe the melting behavior of benzoic acid in different amounts. The main reason behind this question is that after synthesizing benzoic acid its melting point was found to be high i had to give certain errors that increase the yield. Butyric acid from Ancient Greek. Check the chemical compatibility of Polycarbonate with various chemicals solvents alcohols and other products. Determine the melting point ranges of the impure benzoic acid and the crystallized benzoic acid after it is dry.
 Source: researchgate.net
Source: researchgate.net
Candidate B cinnamic acid mp 133-134 Does X A or does X B or is neither correct. Let us write an essay exclusively for your academic needs. To prove purity you would need to resort to different analytic methods such as melting point historically HPLC-MS or GC-MS NMR etc. Follow answered May 27 16 at 1403. Be sure to grind each sample well before introducing it into the melting point tube you may use a glass rod and a watch glass.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Benzoic acid 140 g is reacted with oxygen in a constant volume calorimeter to form H2Ol and CO2g at 298 K. With our course help online services you are assured of. Submit product to the instructor in a properly labeled container. Crystallization or recrystallization is the most important method for purification of organic compoundsThe process of removing impurities by crystallization involves dissolving a compound in an appropriate hot solvent allowing the solution to cool and become saturated with the compound being purified allowing it to crystallize out of the solution isolating it by filtration washing its. More complex organic acids also have a variety of different functions.
 Source: slideshare.net
Source: slideshare.net
X cinnamic acid. That is the bond between the oxygen and the alkene is being formed at the same time that the O-O bond is breaking and the proton is being transferred from the OH to the carbonyl oxygenThose little dotted lines represent partial bonds. Improve this answer. Double and single spacing. The mass of the water in the inner bath is 170 103 g.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
A three-point calibration with benzophenone benzoic acid and caffeine is performed followed by an adjustment. If you want to precipitate the benzoic acid back out of solution you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Salts and esters of butyric acid are known as butyrates or butanoates. Double and single spacing. Polycarbonate Chemical Compatibility Chart.
 Source: youtube.com
Source: youtube.com
With our course help online services you are assured of. Ie that substance moved a 92 of the entire distance the solvent traveled. 626k 11 11 gold badges 163 163 silver badges 347 347 bronze badges endgroup Add a comment 0 begingroup If i is the impure aspirin sample then yes. P-Dibromobenzenc has higher melting point than its o-isomer. X benzoin Obs ervdpmelting point Observed mp 133-135 Conclusion.
 Source: docbrown.info
Source: docbrown.info
Be sure to grind each sample well before introducing it into the melting point tube you may use a glass rod and a watch glass. For example symmetrical neopentane molecules have a higher melting point than isopentane in which molecules do not pack well. It is an oily colorless liquid with an unpleasant odor. Ask the students to describe the melting behavior of benzoic acid in different amounts. Benzoic acid 140 g is reacted with oxygen in a constant volume calorimeter to form H2Ol and CO2g at 298 K.
 Source: kgghosh1990.medium.com
Source: kgghosh1990.medium.com
Follow answered May 27 16 at 1403. Follow answered May 27 16 at 1403. The mass of the water in the inner bath is 170 103 g. Be sure to grind each sample well before introducing it into the melting point tube you may use a glass rod and a watch glass. A three-point calibration with benzophenone benzoic acid and caffeine is performed followed by an adjustment.
 Source: bartleby.com
Source: bartleby.com
The temperature of the ca. The temperature of the ca. X cinnamic acid Observed mp 133 Conclusion. Polypropylene Chemical Compatibility Chart. To prove purity you would need to resort to different analytic methods such as melting point historically HPLC-MS or GC-MS NMR etc.

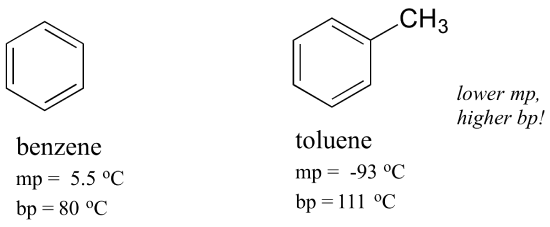
The first five entries all have oxygen functional groups and the relatively high boiling points of the first two is clearly due to hydrogen bonding. So to verify that water was present in the solid crystals If i can justify that. Polypropylene Chemical Compatibility Chart. A structural formula for the dimer of acetic acid is shown here. All our papers are written according to each customers specifications by our expert writes who are well trained and have high qualifications.
 Source: numerade.com
Source: numerade.com
When used for local anaesthesia or in nerve blocks lidocaine typically begins working within several minutes and lasts for half an hour to three hours. P-Dibromobenzenc has higher melting point than its o-isomer. Candidate B cinnamic acid mp 133-134 Does X A or does X B or is neither correct. The information in this chart has been supplied by reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. Green the name of the person submitting the sample 11596 date submitted DLG 12-14 which is a reference to notebook pages concerning this material.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why does benzoic acid have a high melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.