What is the melting point of naphthalene
Home » datasheet » What is the melting point of naphthaleneWhat is the melting point of naphthalene
What Is The Melting Point Of Naphthalene. Take naphthalene on a tile and crush it into a fine powder. The solution was also decolorized twice instead of once. Cloud point CP is the temperature at which wax first becomes visible when the fuel is cooled ASTM D2500 Pour point is the temperature at which the amount of wax out of solution is sufficient to gel the fuel ASTM D97. Melting Point of Naphthalene ºC 76.
 The Melting Point Of Naphthalene Is 80 C And The Room Temperature Is 30 Daily Study Tips From dailystudytips.com
The Melting Point Of Naphthalene Is 80 C And The Room Temperature Is 30 Daily Study Tips From dailystudytips.com
Use examples of dry ice and naphthalene in a Predict-Observe-Explain POE demonstration showing that some substances can change state from a solid to a gas without becoming a liquid. The compounds extracted seem to be slightly impure. Take a capillary tube and close its one end by heating the end in the flame for 2-3 minutes while continuously rotating it. Grant and contract funding is sourced from the US National Institutes of Health the Bill Melinda Gates Foundation The Wellcome Trust EDCTP the South African Medical Research Council the National Research Foundation of South. An acid-base extraction operates on the same principle but can provide a further level of fine -tuning. The surrounding temperature around a storage tank should always.
Some examples are plug.
Instead of using water to recrystallize benzoic acid ethanol was used in 32B part 1. A 173 mg sample of an organic compound a non-electrolyte was ground up with 420 mg of camphor to form a homogeneous mixture melting at 1700 o C. We would like to show you a description here but the site wont allow us. What is the freezing point of an aqueous 100 m NaCl solution. The softness of pure 24 karats gold is usually alloyed. In practice a solid usually melts over a range of temperatures rather than at one specific temperature.
 Source: chemistry.analia-sanchez.net
Source: chemistry.analia-sanchez.net
Dip the open end of the capillary tube in the. Recrystallization of Benzoic Acid was performed according to the method of Gilbert and Morgan 2001 section 32. K f 186 o Cm Assume complete dissociation of the salt a -186 o C b 186 o C c -372 o C d -093 o C e 00 o C 14. Take a capillary tube and close its one end by heating the end in the flame for 2-3 minutes while continuously rotating it. At a temperature of 176F naphthalene sublimes to form vapours.
 Source: dailystudytips.com
Source: dailystudytips.com
A 173 mg sample of an organic compound a non-electrolyte was ground up with 420 mg of camphor to form a homogeneous mixture melting at 1700 o C. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. Take a capillary tube and close its one end by heating the end in the flame for 2-3 minutes while continuously rotating it. Another well-known example of sublimation is naphthalene which is an organic compound. The solution was also decolorized twice instead of once.
 Source: researchgate.net
Source: researchgate.net
To C 59To F - 32 Note. A 173 mg sample of an organic compound a non-electrolyte was ground up with 420 mg of camphor to form a homogeneous mixture melting at 1700 o C. The solution was also decolorized twice instead of once. Cold filter plugging point CFPP is the lowest temperature at which fuel will pass through a fine wire mesh screen. Generally flash point increases with an increase in boiling point.
 Source: slideshare.net
Source: slideshare.net
Naphthalene an organic compound commonly found in pesticides such as mothballs sublimes easily because it is made of non-polar molecules that are held together only by van der Waals intermolecular forces. Naphthalene an organic compound commonly found in pesticides such as mothballs sublimes easily because it is made of non-polar molecules that are held together only by van der Waals intermolecular forces. There are not huge discrepancies in the numbers so this leads me to believe the compounds extracted are the expected compounds. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. An acid-base extraction operates on the same principle but can provide a further level of fine -tuning.
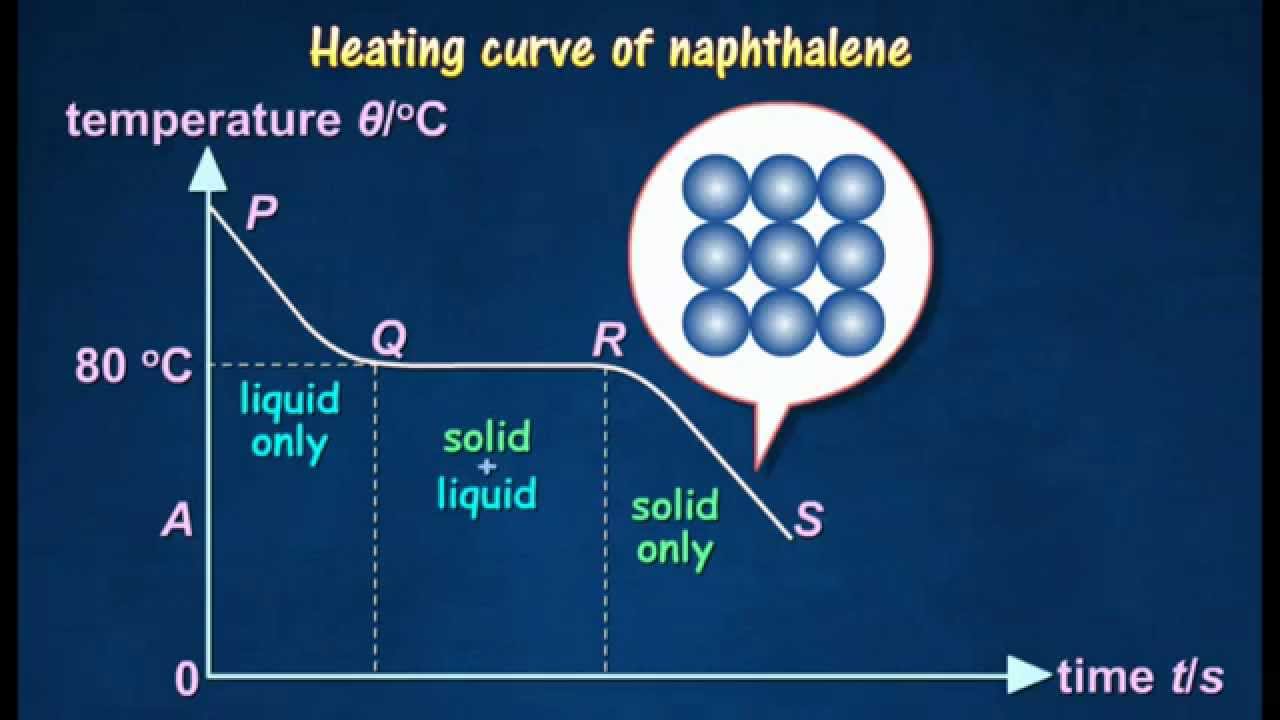 Source: youtube.com
Source: youtube.com
It has a sligtly lower melting point but greater chain flexibility. If the compound melts over a very. Recrystallization of Benzoic Acid was performed according to the method of Gilbert and Morgan 2001 section 32. At a temperature of 176F naphthalene sublimes to form vapours. In this equation T FP is the freezing point depression the change in the freezing point that occurs when the solute dissolves in the solvent – and k f is the molal freezing point depression constant for the solvent.
 Source: snapsolve.com
Source: snapsolve.com
Investigate how students could. The solution was also decolorized twice instead of once. Naphthalene is usually found in pesticides such as mothball. - the Auto-Ignition Temperature is not the same as Flash Point - The Auto. As shown in the figure below firmly hold the closed end of the capillary tube between your finger and thumb.
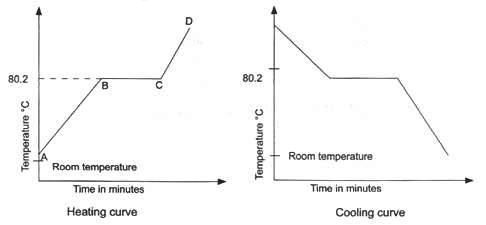 Source: sukachem.blogspot.com
Source: sukachem.blogspot.com
K f 186 o Cm Assume complete dissociation of the salt a -186 o C b 186 o C c -372 o C d -093 o C e 00 o C 14. Use examples of dry ice and naphthalene in a Predict-Observe-Explain POE demonstration showing that some substances can change state from a solid to a gas without becoming a liquid. For this reason it is more useful to speak of a melting point range. K f 186 o Cm Assume complete dissociation of the salt a -186 o C b 186 o C c -372 o C d -093 o C e 00 o C 14. Although the term melting point is usually used what is meant is melting point range.
 Source: ddbst.com
Source: ddbst.com
At a temperature of 176F naphthalene sublimes to form vapours. Naphthalene is soluble in methanol and insoluble in water. The higher the freezing point constant the higher the freezing depression point hence the higher the accuracy. Another well-known example of sublimation is naphthalene which is an organic compound. MeltingBoiling point Alkane Alkene Alkyne 2 Melting point ethane-183 ethene-169 ethyne-807 Boiling point ethane-89 ethene-104 ethyne-847 3 Melting point propane-190 propene-185 propyne-1027 Boiling point propane-42 propene-47 propyne-232 4 Melting point butane-138 1-butene-1853 1-butyne-1257 Boiling point butane-05 1-butene-62.
 Source: shaalaa.com
Source: shaalaa.com
An acid-base extraction operates on the same principle but can provide a further level of fine -tuning. In practice a solid usually melts over a range of temperatures rather than at one specific temperature. Instead of using water to recrystallize benzoic acid ethanol was used in 32B part 1. - the Auto-Ignition Temperature is not the same as Flash Point - The Auto. Naphthalene an organic compound commonly found in pesticides such as mothballs sublimes easily because it is made of non-polar molecules that are held together only by van der Waals intermolecular forces.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It finds many uses as an engineering thermoplastic particularly in electrical engineering and in automotive construction. Its maximum use temperature is 390 - 410 K slightly lower than that of PET. PBT crystallizes much faster than PET and competes with PET in engineering plastics applications. Add some of this hot solvent to. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the melting point of naphthalene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.