What is the melting point of dichloromethane
Home » datasheet » What is the melting point of dichloromethaneWhat is the melting point of dichloromethane
What Is The Melting Point Of Dichloromethane. -4C 25 F s Ignition temperature. 12 Vol Upper. No boiling point at atmospheric conditions pure as. 398-40C 1036-104F Flash Point.
 Dichloromethane Formula From softschools.com
Dichloromethane Formula From softschools.com
BUCHIs melting point analyzers fully automate the rather time-consuming process of melting point and boiling point determination and provide highly accurate results. It can however be cooled down to 78 C 108 F using a dry iceacetone bath Diethyl ether CH 3 CH 2 OCH 2. 967 C 1421 F. The organic solvens t must also be volatile easily evaporated so it can be removed by evaporation at the end of the process. Most pure organic compounds melt over a narrow temperature range of 1-2 C. 12-dichloromethane 250 ethyl acetate 250 methanol 250 3.
5561 C 10330 F Explosion limits.
Would not flash Flash point method and additional flammability data. 12-dichloromethane 250 ethyl acetate 250 methanol 250 3. The melting point is the highest temperature at which crystallization may occur. ILO International Chemical Safety Cards. For example the melting point of ice frozen water is 0 C. Carefully note your observations on what the sample looks like as it heats up.
 Source: acs.org
Source: acs.org
The solution of these dissolved compounds is referred to as the extract. BUCHIs melting point analyzers fully automate the rather time-consuming process of melting point and boiling point determination and provide highly accurate results. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. 12-dichloromethane 250 ethyl acetate 250 methanol 250 3. 256 gL 15 C 175 gL 25 C 158 gL 30 C 52 gL 60 C Solubility.
This procedure consists of two steps. Replacing dichloromethane is advantageous when conditions require higher boiling solvents since trifluorotoluene boils 62 C higher than. In selecting a solvent consider that like likes like. Product Name Dichloromethane anhydrous Cat No. In selecting a good recrystallization solvent one should also consider flammability toxicity and expense.
 Source: byjus.com
Source: byjus.com
CHEMICAL PRODUCT AND COMPANY IDENTIFICATION. -4C 25 F s Ignition temperature. No boiling point at atmospheric conditions pure as. Extraction is a method used for the separation of organic compound from a mixture of compound. 69 C pure ai 671 C for TGAI at atmospheric pressure Boiling point.
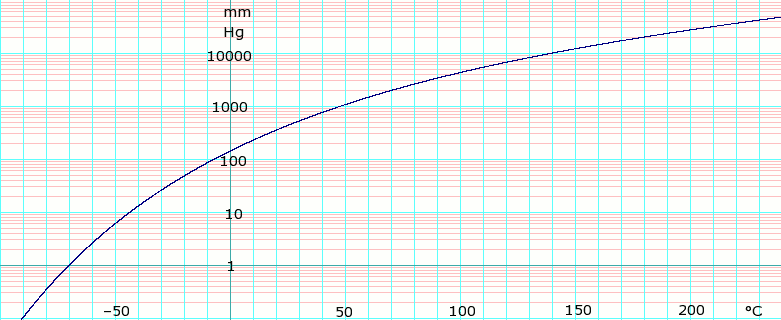 Source: en.wikipedia.org
Source: en.wikipedia.org
-139F -95C VAPOUR PRESSURE. Most pure organic compounds melt over a narrow temperature range of 1-2 C. The dielectric constants for dichloromethane and trifluorotoluene are 904 and 918 respectively indicating similar solvating properties. 013 kPa. The melting point is the highest temperature at which crystallization may occur.
 Source: en.wikipedia.org
Source: en.wikipedia.org
As the compound is highly volatile in nature it can cause acute inhalation hazards. It is also a temperature at which a solid crystal turns into a liquid. In selecting a good recrystallization solvent one should also consider flammability toxicity and expense. Would not flash Flash point method and additional flammability data. The melting point is the highest temperature at which crystallization may occur.

In the case of Caffeine extraction from tea powder the solubility of caffeine in water. 398-40C 1036-104F Flash Point. 12 Vol Upper. Looking up the literature mp of caffeine prior to measuring the mp will give you an idea of the approximate temperature to be expected. 2 has a dipole moment of 193 D and a boiling point of 52C.
 Source: sciencemadness.org
Source: sciencemadness.org
Why is the boiling point of dichloromethane 92º higher than that of difluoromethane. Most pure organic compounds melt over a narrow temperature range of 1-2 C. It is also used as a degreasing agent. News See more News Events. This procedure consists of two steps.

Would not flash Flash point method and additional flammability data. Solvent formula polarity boiling point 0 C water H2O very polar 100 ethanol CH3CH2OH polar 78 methanol CH3OH polar 65 dichloromethane CH2Cl2 slightly polar 40 diethyl ether CH3CH22O slightly polar 35 Organic compounds with one polar functional group and a low number of carbon atoms such. -139F -95C VAPOUR PRESSURE. Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. 1765 K Boiling point.
 Source: softschools.com
Source: softschools.com
31290 K at 760 mmHg. 40 d 133 which is immiscible with water it is widely used as a solvent a paint stripper and for the removal of caffeine from coffee and tea. Which of the following explains why dichloromethane has the. Ethyl acetate CH 3 COOC 2 H 5 is an excellent solvent with boiling point 78 C 172 F. 12 Vol Upper.
 Source: en.wikipedia.org
Source: en.wikipedia.org
No boiling point at atmospheric conditions pure as. Dissolve in approximately 50 mL dichloromethane. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. -139F -95C VAPOUR PRESSURE. 396 C 1033 F.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the melting point of dichloromethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.