What is the boiling point of kcl
Home » datasheet » What is the boiling point of kclWhat is the boiling point of kcl
What Is The Boiling Point Of Kcl. Which of species can exhibit hydrogen bonding among themselves. G Flash point -170 C 14 F - closed cup h Evaporation rate No data available i Flammability solid gas No data available. Which of the following has the highest boiling point. Potassium Chloride is a metal halide composed of potassium and chloride.

Molten salts have high boiling points low viscosity low vapor pressure and high volumetric heat capacities. 0 o C 4 o C 96 o C 100 o C At. Pointfreezing point Melting pointrange. Melting point - the temperature at which a solid turns into a liquid. Ethyl ether and petroleum ether are the most commonly used solvents but pentane and hexane are. HF HCl HBr HI e.
LiH HF At which temperature does water have its greatest density.
Which of the following has the highest boiling point. C 6 H 6. In addition to the above considerations a solvent should also be inexpensive have a relatively low boiling point so that it can easily be removed by evaporation be non-toxic and be nonflammable for safety reasons. 2 A higher heat capacity corresponds to a smaller storage tank volume. Which of species can exhibit hydrogen bonding among themselves. B 2 H 6.
 Source: quora.com
Source: quora.com
In addition to the above considerations a solvent should also be inexpensive have a relatively low boiling point so that it can easily be removed by evaporation be non-toxic and be nonflammable for safety reasons. 0 o C 4 o C 96 o C 100 o C At. In choosing the chemical mixture it is advantageous to have the lowest possible melting point and highest boiling point to maximize the available temperature range for the molten salt. F Initial boiling point and boiling range 56 C 133 F at 1013 hPa - lit. KCl is used as a fertilizer in medicine in scientific applications and.
 Source: youtube.com
Source: youtube.com
KCl is used as a fertilizer in medicine in scientific applications and. In choosing the chemical mixture it is advantageous to have the lowest possible melting point and highest boiling point to maximize the available temperature range for the molten salt. HF HCl HBr HI e. It is a strong oxidizing agent and its most important application is in safety matches. Ethyl ether and petroleum ether are the most commonly used solvents but pentane and hexane are.
 Source: youtube.com
Source: youtube.com
Melting point - the temperature at which a solid turns into a liquid. In addition to the above considerations a solvent should also be inexpensive have a relatively low boiling point so that it can easily be removed by evaporation be non-toxic and be nonflammable for safety reasons. Reduce and denature the samples by boiling the lysates in Laemmli Buffer at 95-100C for 5 minutes. It is a strong oxidizing agent and its most important application is in safety matches. F Initial boiling point and boiling range 56 C 133 F at 1013 hPa - lit.

Potassium Chloride is a metal halide composed of potassium and chloride. Boiling point - the temperature at which a liquid turns into a gas. In choosing the chemical mixture it is advantageous to have the lowest possible melting point and highest boiling point to maximize the available temperature range for the molten salt. Pointfreezing point Melting pointrange. 0 o C 4 o C 96 o C 100 o C At.
 Source: youtube.com
Source: youtube.com
Which of species can exhibit hydrogen bonding among themselves. C 6 H 6. This step should only be skipped if the antibody datasheet recommends non-reducing or non-denaturing conditions. In other applications it is mostly obsolete and has been. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
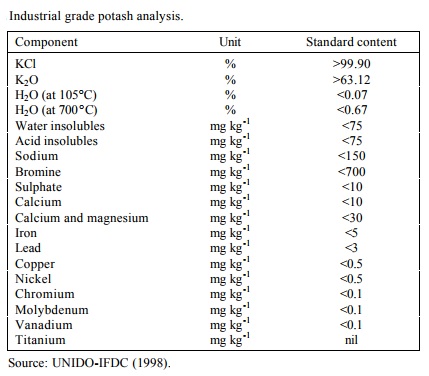 Source: chemicalbook.com
Source: chemicalbook.com
Ne Ar Kr Xe d. B 2 H 6. Melting point - the temperature at which a solid turns into a liquid. The solid dissolves readily in water and its solutions have a salt-like tastePotassium chloride can be obtained from ancient dried lake deposits. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
HF HCl HBr HI e. Which of species can exhibit hydrogen bonding among themselves. Melting point - the temperature at which a solid turns into a liquid. In other applications it is mostly obsolete and has been. 0 o C 4 o C 96 o C 100 o C At.

Reduce and denature the samples by boiling the lysates in Laemmli Buffer at 95-100C for 5 minutes. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. Which of species can exhibit hydrogen bonding among themselves. Boiling point - the temperature at which a liquid turns into a gas. Ne Ar Kr Xe d.

G Flash point -170 C 14 F - closed cup h Evaporation rate No data available i Flammability solid gas No data available. Pointfreezing point Melting pointrange. 2 A higher heat capacity corresponds to a smaller storage tank volume. Which of species can exhibit hydrogen bonding among themselves. Ethyl ether and petroleum ether are the most commonly used solvents but pentane and hexane are.
 Source: youtube.com
Source: youtube.com
Pointfreezing point Melting pointrange. Ne Ar Kr Xe d. Boiling point - the temperature at which a liquid turns into a gas. Potassium chloride KCl or potassium salt is a metal halide salt composed of potassium and chlorineIt is odorless and has a white or colorless vitreous crystal appearance. Potassium Chloride is a metal halide composed of potassium and chloride.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the boiling point of kcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.