What is the boiling point of fluorine
Home » datasheet » What is the boiling point of fluorineWhat is the boiling point of fluorine
What Is The Boiling Point Of Fluorine. 1673 C 2691 F. Fluorine is a naturally-occurring pale yellow-green gas with a sharp odor. The first recorded use of a fluorine compound dates to around 1670 to a set of instructions for etching glass that called for Bohemian emerald CaF. Note that these points are associated with the standard atmospheric pressure.
 Q1itufghdbn8em From
Q1itufghdbn8em From
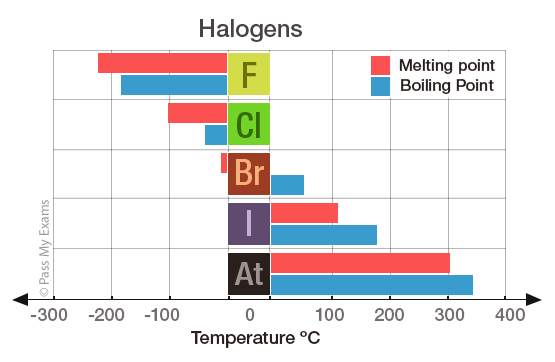
You can easily determine what the boiling or freezing point of any solution will be using a simple equation. Fluorine is an univalent poisonous gaseous halogen. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Just like how the strength of the bonds between atoms affect the Melting Point the boiling point depends on the heat energy required to create a transition from liquid to gaseous state. Boiling point of Fluorine is -1881C. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the.
Melting point of Fluorine is -2198C.
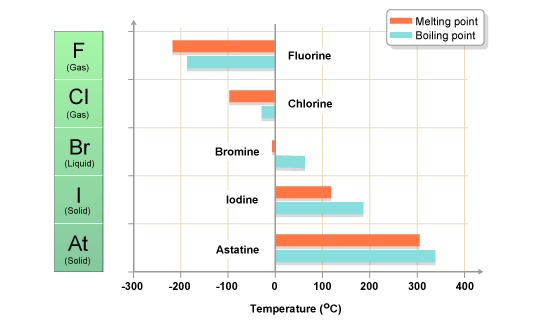
The boiling point of aluminium is much higher than magnesiums - as you would expect. Sodium fluoride dissolves easily in water but calcium fluoride does not. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. This means that it will be solid at room temperature. Have a look at this table with the elements of the periodic table arranged in order of increasing boiling points. This list contains the 118 elements of chemistry.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
Standard potential - 287 V. As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. Boiling Point Date of Discovery Crystal Structure. Through wind-blown soil fluorides are released into the air. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the.
 Source: material-properties.org
Source: material-properties.org
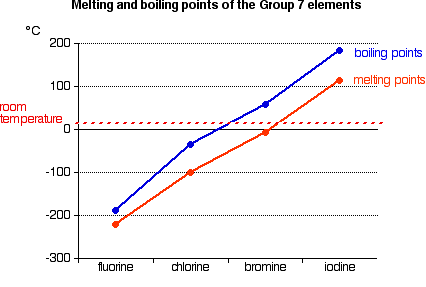
The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. The boiling point of aluminium is much higher than magnesiums - as you would expect. 0136 nm -1. In general boiling is a phase change of a substance from the liquid to the gas phase. A halide is a.
 Source: chemguide.co.uk
Source: chemguide.co.uk
If you come across an explanation for the very small increase in melting point from magnesium to aluminium in terms of the strength of the metallic bond you should be very wary of it unless it also explains why despite that the boiling point of aluminium is much higher than that of magnesium. The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. Fluorine occurs naturally in the crust of the earth where it is present in rocks coal and clay. Most of their boiling points are higher than the ten electron compounds neon and methane but fluorine is an exception boiling 25º below methane. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Source: britannica.com
Source: britannica.com
Fluorine can form ionic bonds with some elements such as carbon and boron and neon does not tend to form any bonds at all. The anomalous behavior of fluorine may be attributed to its very high. As the molality changes it affects the boiling point and freezing point also known as the melting point of the solution. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Alkali Metals Alkaline Earth Metals Transition Metals Other Metals Metalloids Non-Metals Halogens Noble Gases Rare Earth Elements The halogens are five non-metallic elements found in group 17 of the periodic table.
 Source: rsc.org
Source: rsc.org
7837 C 1731 F Boiling point of methanol. The Earths crust contains 950 parts per million of fluorine. A halide is a. The chemical elements of the periodic chart sorted by. Standard potential - 287 V.
 Source:
Source:
Melting point - the temperature at which a solid turns into a liquid. Note that these points are associated with the standard atmospheric pressure. The temperature at. The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. Note that these points are associated with the standard atmospheric pressure.
 Source: lizzyfluorine.weebly.com
Source: lizzyfluorine.weebly.com
257 K Thermochemistry Std enthalpy of. Use a table to look up. The remaining examples in the table conform to the correlation of boiling point with total electrons and number of nuclei but fluorine containing molecules remain an exception. The temperature at. Note the molality m of the solution.
 Source: slideplayer.com
Source: slideplayer.com
Note the molality m of the solution. P Density g cm 3 0001553. Most of their boiling points are higher than the ten electron compounds neon and methane but fluorine is an exception boiling 25º below methane. Boiling point of water. Sodium fluoride dissolves easily in water but calcium fluoride does not.
 Source: daviddarling.info
Source: daviddarling.info
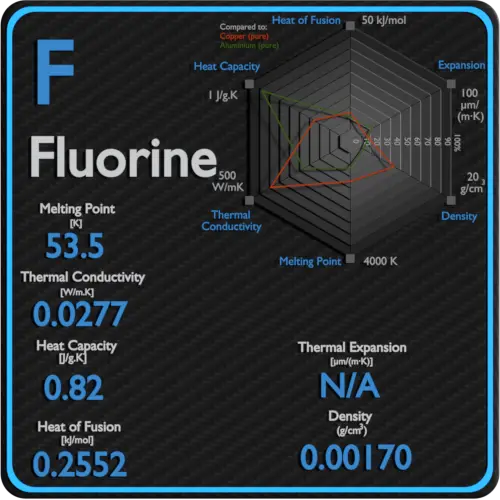
Fluorine is a chemical element with the symbol F and atomic number 9. The elemenents of the periodic table sorted by boiling point. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Fluorine occurs naturally in the crust of the earth where it is present in rocks coal and clay. 18811C 3066F 8504 K Block.
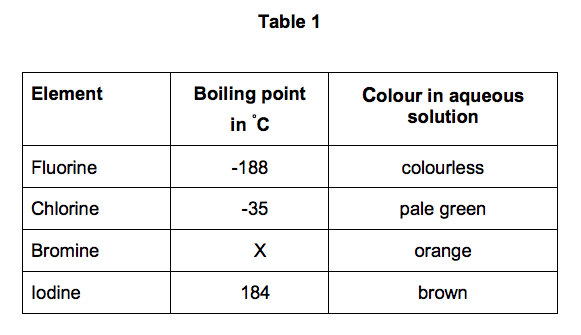 Source: elevise.co.uk
Source: elevise.co.uk
The temperature at. 3732 K Boiling point of ethanol. Fluorine also combines with hydrogen to make hydrogen fluoride a colorless gas. Fluorine is the most reactive of all elements and no chemical substance is capable of freeing fluorine from any of its compounds. Melting and boiling points of 3d metals are generally higher than s block elements.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the boiling point of fluorine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.