What is the boiling point of dichloromethane
Home » datasheet » What is the boiling point of dichloromethaneWhat is the boiling point of dichloromethane
What Is The Boiling Point Of Dichloromethane. DCM is used as a solvent in the food industry and as a paint remover. National Toxicology Program Chemical Repository Database. Would not flash Flash point method and additional flammability data are found in Section 5 10. Speed up this process is the following.

A wide variety of solvents like dichloromethane DCM pure or mixed with acetone or hexane and acetonehexane mixtures can be used. The attraction between the alkyl halide molecules is stronger than the attraction between the alkyl halide and water. Research Triangle Park North Carolina. 2989 44 39 Acetic acid. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. During the water process method you place the coffee beans in water and heat to around boiling point.
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol.
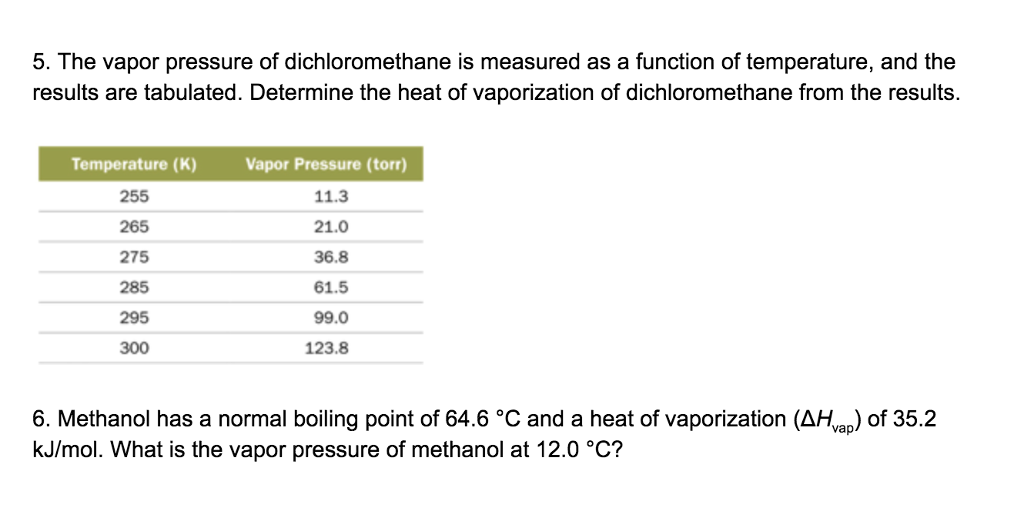
We also acknowledge previous National Science Foundation support under grant numbers 1246120 1525057 and 1413739. The minimum time needed for a regular Soxhlet extraction is normally 8 hours. These alcohols form hydrogen bond. 5561 C 10330 F Explosion limits. Would not flash Flash point method and additional flammability data are found in Section 5 10. When dichloromethane and water mix together they separate into two layers.
 Source: future4200.com
Source: future4200.com
A wide variety of solvents like dichloromethane DCM pure or mixed with acetone or hexane and acetonehexane mixtures can be used. Physical and chemical properties Appearance. We also acknowledge previous National Science Foundation support under grant numbers 1246120 1525057 and 1413739. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. Product Name Dichloromethane anhydrous Cat No.

The use of nonpolar solvents only is not recommended. Why is the boiling point of dichloromethane 92º higher than that of difluoromethane. We also acknowledge previous National Science Foundation support under grant numbers 1246120 1525057 and 1413739. The boiling point also increases as a result of increasing the size of the halogen as well as increasing the size of the carbon chain. The LibreTexts libraries are Powered by MindTouch and are supported by the Department of Education Open Textbook Pilot Project the UC Davis Office of the Provost the UC Davis Library the California State University Affordable Learning Solutions Program and Merlot.
 Source: chegg.com
Source: chegg.com
One useful trick to. The boiling point is specific for the given substanceFor example the boiling point of. The boiling point at atmospheric pressure. Speed up this process is the following. 25 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: softschools.com
Source: softschools.com
12 Vol Upper. 12 Use the vacuum pump to remove residual solvent. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. 398-40C 1036-104F Flash Point. Would not flash Flash point method and additional flammability data are found in Section 5 10.
 Source: en.wikipedia.org
Source: en.wikipedia.org
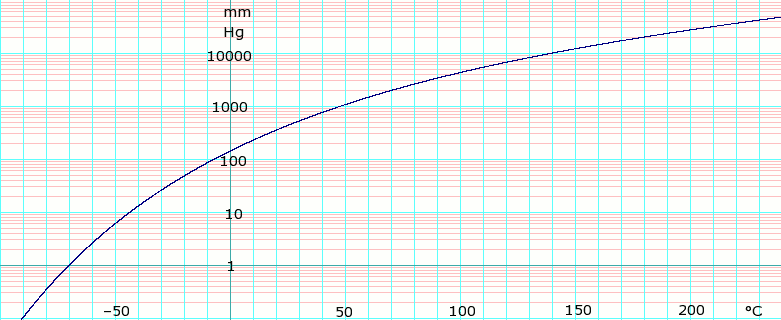
CAMEO Chemicals-42 F closed cup Lewis RJ. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. 398-40C 1036-104F Flash Point. 140F 40C MELTING POINT. Research Triangle Park North Carolina.
 Source: acs.org
Source: acs.org
Boiling point of DCM. Alkyl halides have little to no solubility in water in spite of the polar carbon-halogen bond. Organic compounds with one polar functional group and a low number of carbon atoms such as methanol ethanol and n-propanol are highly soluble miscible in water. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. During the water process method you place the coffee beans in water and heat to around boiling point.
Would not flash Flash point method and additional flammability data are found in Section 5 10. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. 1843 369 596 587 K b K f. When dichloromethane and water mix together they separate into two layers. 12 Vol Upper.
 Source: en.wikipedia.org
Source: en.wikipedia.org
12 Vol Upper. With stronger intermolecular attraction of course CH 2F 2 will have a lower boiling point. Aqueous material from the dichloromethane solution at this point. Product Name Dichloromethane anhydrous Cat No. Higher boiling solvents are more effectively removed by concentrating adding dichloromethane then repeating once more.
 Source: researchgate.net
Source: researchgate.net
Place one Boileezer into the beaker. Aqueous material from the dichloromethane solution at this point. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. Research Triangle Park North Carolina. Fuels - Densities and Specific Volumes - Densities and specific volumes fuels like anthracite butane gasoil diesel coke oil wood and more.

Hazards of using Dichloromethane. 1843 369 596 587 K b K f. The compound is also used in the production of aerosol formulations. Hazards of using Dichloromethane. Finally you put the beans in the.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the boiling point of dichloromethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.