What is the boiling point of cyclohexane
Home » datasheet » What is the boiling point of cyclohexaneWhat is the boiling point of cyclohexane
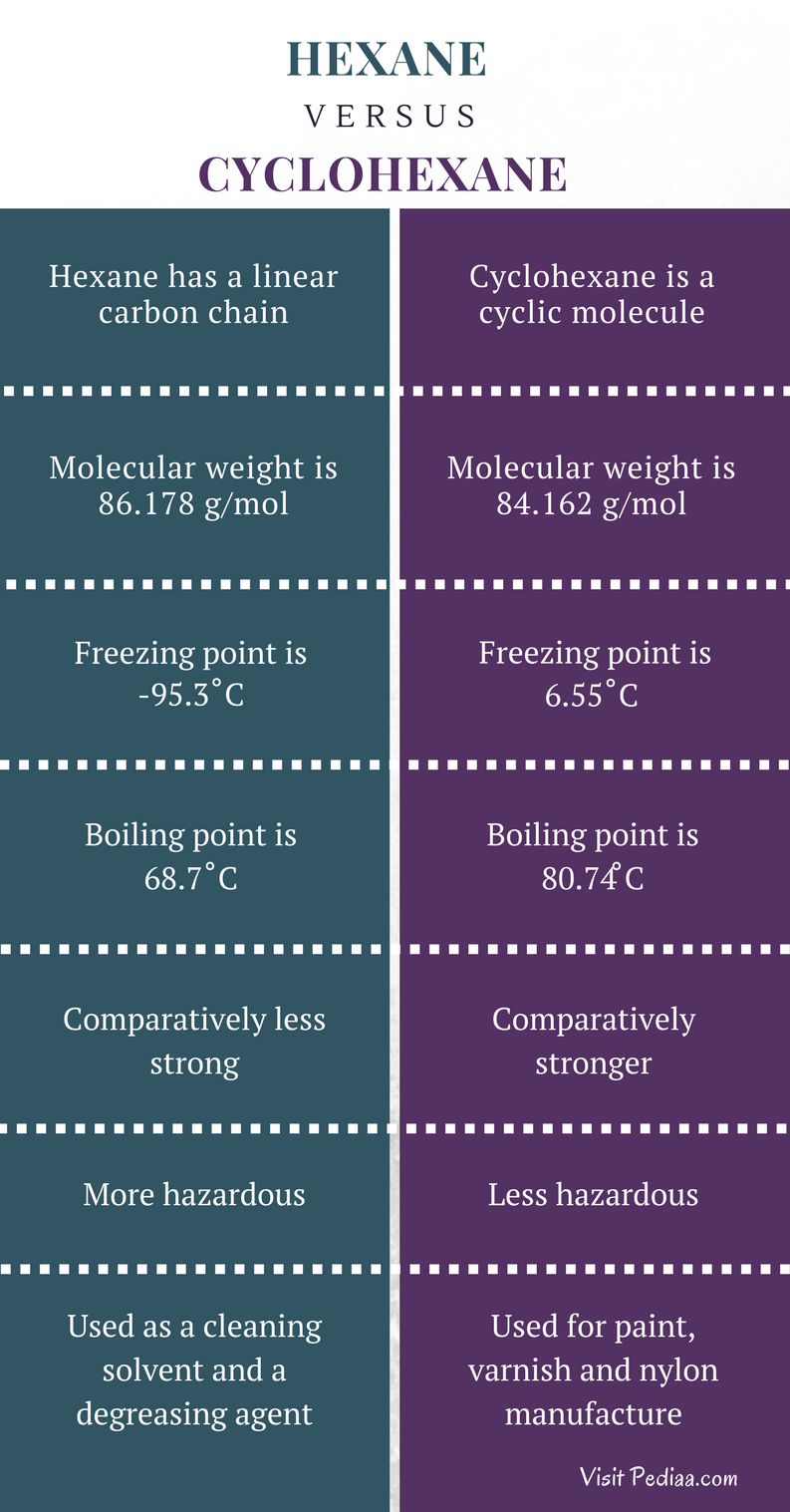
What Is The Boiling Point Of Cyclohexane. Cyclohexane is a cycloalkane with the molecular formula C 6 H 12Cyclohexane is non-polarCyclohexane is a colorless flammable liquid with a distinctive detergent-like odor reminiscent of cleaning products in which it is sometimes usedCyclohexane is mainly used for the industrial production of adipic acid and caprolactam which are precursors to nylon. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Alkanes consist of four carbon atoms at standard temperatures. Water boils at 373 K and ethanol boils at.
 Solved Explain Why The Boiling Point Of Your Cyclohexane Chegg Com From chegg.com
Solved Explain Why The Boiling Point Of Your Cyclohexane Chegg Com From chegg.com
Dioxane methanol ethanol nitric acid nitromethane pyridine phosphorous oxychloride. Bromine chloroform cyclohexane ethyl acetate triethylamine. Stability and Reactivity Stability. The boiling point is specific for the given substanceFor example the boiling point of. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Highly branched vs.
3 Chemical and Physical Properties Expand this section.
Note that under vacuum the BP of a liquid will be lower than the BP at atmospheric pressure. CI is defined in terms of Mean Average Boiling Point Tb and specific gravity SG at 60F as shown in Equation 10. Acetone CH 3 COCH 3. Alkanes comprising of more than three carbon atoms are capable of. Freezing point of the fluid 79 C has made this fluid the only air- breathing missile fuel used by the United States at the present time. The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent.

If this all seems rather ambiguous contradictory and imprecise well you have a point. 95 20C 68F Evaporation Rate BuAc1. Alkanes comprising of more than three carbon atoms are capable of. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. Stability and Reactivity Stability.
 Source: chegg.com
Source: chegg.com
Suppose you are going to. Of course additional criteria must also be satisfied before the. C I 87 552 T b 4737 S G 4568. The boiling point is specific for the given substanceFor example the boiling point of. Water boils at 373 K and ethanol boils at.

The boiling point is specific for the given substanceFor example the boiling point of. If this all seems rather ambiguous contradictory and imprecise well you have a point. Δt i K b m. 26 Ether 1 10. The solvents must be miscible in one another.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
26 Ether 1 10. The definition of the boiling point of a liquid in an open container then is the temperature at which its vapor pressure equals atmospheric pressure. Branched more sphere-like - lower surface area lower boiling point. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. Maximum Boiling Azeotropes or Positive Azeotrope.
 Source: pediaa.com
Source: pediaa.com
2 We utilize this formula. Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model. The solvents must be miscible in one another. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration. Solvent formula polarity boiling point 0C water H2O very polar 100 ethanol CH3CH2OH polar 78 methanol CH3OH polar 65.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
An isomer of isooctane gasoline with melting point 95 C. Alkanes consist of four carbon atoms at standard temperatures. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester. DMSO nitrobenzene octanoles sulfuric acid. Its not a straightforward topic.

Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Similarly an azeotropic mixture that has a boiling point lesser than its constituents is known as minimum boiling azeotropes. The boiling point and boiling point range have been used as criteria in confirming both the identity and purity of a substance. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. The mole fraction of component 1 in the mixture can be represented by the symbol x 1.
To achieve the goal of producing a provisional or preliminary physical property model measurements of chemical composition thermal decomposition density viscosity thermal conductivity. Highly branched vs. The majority of solvent cleaning work is performed in equipment of two types relative to flash point either an open tank or a closed tank. The solvents must be miscible in one another. Find more information about azeotropes at Wikipedia Find more information about the method of prediction in the About.
 Source: en.wikipedia.org
Source: en.wikipedia.org
According to this CI scale all n-paraffins have a CI value of 0 while cyclohexane the simplest naphthene has a CI value of 50 and benzene has a CI value of 100. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. According to this CI scale all n-paraffins have a CI value of 0 while cyclohexane the simplest naphthene has a CI value of 50 and benzene has a CI value of 100. Each point on this line therefore describes the vapor. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding.

147 g 16214 gmol 0647 kg 140127 m. If the length is more the boiling melting point will be higher. Consider for example ethanol consisting of a weight concentration of approximately ninety-five per cent and four per cent of water. Alkanes consist of four carbon atoms at standard temperatures. Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the boiling point of cyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.