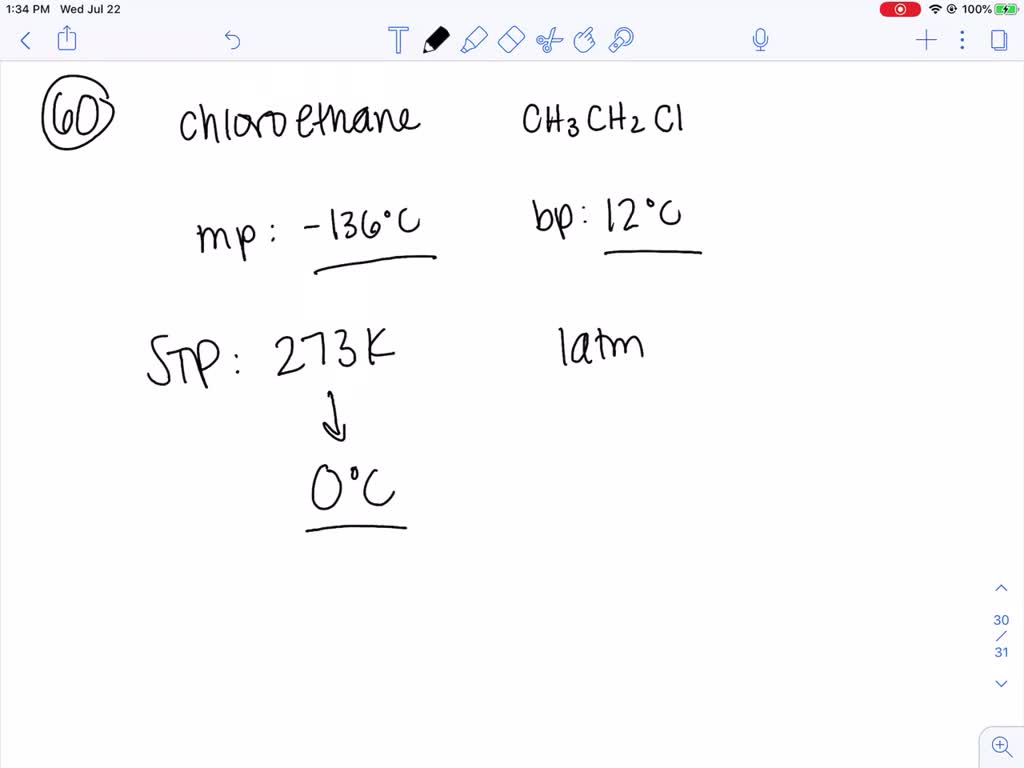
What is the boiling point of chloroethane
Home » datasheet » What is the boiling point of chloroethaneWhat is the boiling point of chloroethane
What Is The Boiling Point Of Chloroethane. The solution of n-hexane and n-heptane bromoethane and chloroethane benzene and toluene chlorobenzene and bromobenzene etc. 100C 212F Flash point. The patterns in boiling point reflect the patterns in intermolecular attractions. A Intermolecular distances are short b Intermolecular forces are weak c Constituent particles have fixed positions d Solids oscillate about their mean positions Ansb.
 Chloroethane Alchetron The Free Social Encyclopedia From alchetron.com
Chloroethane Alchetron The Free Social Encyclopedia From alchetron.com
121 to 110 F. O 10 M NaOH b 10 M Na 2 SO 4 c 10 M NH 4 NO 3 d 10 M KNO 3. 61 to 68 F. Explosive limits Not applicable. Chloroethane however has rather large dipole interactions because of the Cl-C bond. Methyl ethyl ether.
Ethyl chloride Chloroethane 75003 Ethylene dibromide Dibromoethane 106934 Ethylene dichloride 12 -Dichloroethane 107062 Ethylene glycol 107211 Ethylene imine Aziridine 151564 Ethylene oxide 75218 Ethylene thiourea 96457 Ethylidene dichloride 11 -Dichloroethane 75343.
The boiling points of the alcohols increase as the number of carbon atoms increases. It should not be confused with the similar 111-trichloroethane which is commonly known as chlorothene. Very Short Answer Questions. That means that it take 41 kJ to change 1 mole of water into steam. Most of the dilute solutions behave as ideal solutions. Temperature K A B C Reference Comment.
 Source: numerade.com
Source: numerade.com
This is the enthalpy change when 1 mole of the liquid converts to gas at its boiling point with a pressure of 1 bar 100 kPa. The atmospheric pressure at sea level is 760 mmHg. Methyl ethyl ether. 5 - A type of atom which spontaneously undergoes radioactive decay. Use the BACK button on your browser to return to this page.
 Source: toppr.com
Source: toppr.com
B 10 M Na 2 SO 4. Older sources might quote 1 atmosphere rather than 1 bar For water the enthalpy change of vaporisation is 41 kJ mol-1. Must ALWAYS be stored in approved flammable storage cabinet re. 100C 212F Flash point. Fall into this category.
 Source: toppr.com
Source: toppr.com
188 to 194 K Boiling point. 61 to 68 F. Class IB Liquid Flammable Vapor PEL Flash Boiling Point Point o F o F Limits Density ppm Air. Ethyl methanoate has some dipole-dipole interactions but cannot form a hydrogen bond. Major product of the following reaction is _____ ceCH3-CH2-Mg-Br NH3 - CH 3-CH 2-Mg-NH 2.
This is due to the combined strength of so many hydrogen bonds forming between oxygen atoms of one alcohol molecule and the hydroxy H atoms of another. The interaction is therefore stronger leading to a higher boiling point. 16 to 20 C. The process of separation of one liquid from another liquid binary mixture having different boiling points by distillation is called fractional distillation. 289 to 293 K Solubility in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
With its boiling point of 33C cyclopropane is a gas at room temperature. Assertion Reason Solid State QNo1 Which of the following is not a characteristic property of Solids. Chloroethane however has rather large dipole interactions because of the Cl-C bond. Multiple choice questions Q 7. At the boiling point of a liquid the liquids vapor pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid.
 Source: alchetron.com
Source: alchetron.com
Ethyl Chloride Chloroethane -58 54 38 154 22 1000 Ethyl Ether Ether -49 95 19 360 26 400 Isopentane. At the boiling point of a liquid the liquids vapor pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid. You may recall that boiling point is a function of intermolecular interactions which was discussed in the chapter on solutions and colloids. Shows all the covalent bonds present in a molecule Functional group is an atom or. Since the atmospheric pressure at higher elevations is lower than at sea level the boiling point of water decreases as the elevation increases.
 Source: youtube.com
Source: youtube.com
Modifications Pollutants removed from the list of hazardous air pollutants. 4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC. With its boiling point of 33C cyclopropane is a gas at room temperature. Same chemical properties. Van der Waals dispersion forces.

Most of the dilute solutions behave as ideal solutions. Hydrogen bonding raises the boiling point of alcohols. Same chemical properties. SCERT Maharashtra Question Bank solutions for 12th Standard HSC Chemistry Maharashtra State Board 2021 Chapter 10 Halogen DerivativesVery Short. With its boiling point of 33C cyclopropane is a gas at room temperature.
 Source: toppr.com
Source: toppr.com
B 10 M Na 2 SO 4. The IUPAC name is trichloroetheneIndustrial abbreviations include TCE trichlor Trike Tricky and tri. Use the BACK button on your browser to return to this page. Most of the dilute solutions behave as ideal solutions. Explosive limits Not applicable.
 Source: meritnation.com
Source: meritnation.com
Temperature K A B C Reference Comment. Chloroethane however has rather large dipole interactions because of the Cl-C bond. Fall into this category. A Intermolecular distances are short b Intermolecular forces are weak c Constituent particles have fixed positions d Solids oscillate about their mean positions Ansb. The boiling points of the alcohols increase as the number of carbon atoms increases.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the boiling point of chloroethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.