Weight and boiling point of n heptane
Home » datasheet » Weight and boiling point of n heptaneWeight and boiling point of n heptane
Weight And Boiling Point Of N Heptane. Since it is stated that O2 is in excess n-heptane is therefore a limiting reactant. 802 Ammonia aqua 25 0. N-Heptane C7H16 Application Specific n-Heptane. Flupyradifurone Technical Information 7 Solvent gL n-heptane 00005 toluene 37 12-dichloromethane 250 ethyl acetate 250 methanol 250 3.
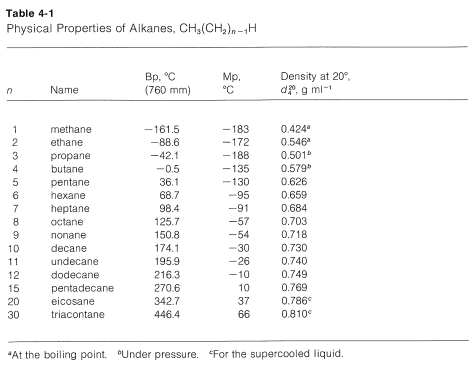 4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
4 2 Physical Properties Of Alkanes And The Concept Of Homology Chemistry Libretexts From chem.libretexts.org
Since it is stated that O2 is in excess n-heptane is therefore a limiting reactant. National Toxicology Program Chemical Repository Database. EFSA Scientific Publications 5 Verified data used for regulatory purposes-Degradation point C 355. At the temperature approximately 1200C the enstatite steadily. Coefficents calculated by NIST from authors data. N-heptane is a clear colorless liquids with a petroleum-like odor.
It has a role as a non-polar solvent and a plant metabolite.
The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents a positive azeotrope or greater than the boiling point of any of its constituents a negative azeotrope. For example n-C 6 F 14 molecular weight 338 and n-C 7 F 16 molecular weight 388 boil at 57C and 82C respectively whereas n-hexane molecular weight 86 and n-heptane molecular weight 100 boil at 69C and 98C respectively. The combustion of n-heptane is C7H16 11O2 7CO2 8H2O Ten 10 kg of n-heptane is reacted with an excess amount of O2 and 144 kg of CO2 is formed. Academiaedu is a platform for academics to share research papers. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Additional Application Specific n.
 Source: researchgate.net
Source: researchgate.net
Flupyradifurone Technical Information 7 Solvent gL n-heptane 00005 toluene 37 12-dichloromethane 250 ethyl acetate 250 methanol 250 3. Distinct solvent-like odour TGAI weak not characteristic pure AI Melting. Academiaedu is a platform for academics to share research papers. Boiling point C Decomposes before boiling. Glycerine has boiling point of 90C which increases its heat carrying capacity.
 Source: thermopedia.com
Source: thermopedia.com
A5 A EU regulatory and evaluation data as published by EC EFSA RAR DAR Conclusion dossiers EMA eg. A5 A EU regulatory and evaluation data as published by EC EFSA RAR. Coefficents calculated by NIST from authors data. Thermodynam 1972 4 773-782. N-Heptane C7H16 Application Specific n-Heptane.
 Source: en.wikipedia.org
Source: en.wikipedia.org
EU Annex III PIC DGD EU - Pesticides database. So an 87-octane gasoline has the same knock resistance as a mixture of 87 isooctane and 13 n-heptane. EU Annex III PIC DGD EU - Pesticides database. Calculate the conversion percentage of n-heptane. 311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: patents.google.com
Source: patents.google.com
A well-known example of a positive azeotrope is 9563 ethanol and 437 water by mass which boils at 782 C. Thermodynamic properties of standard n-heptane from 155 to 270K and of 22-dichloropropane from 135 to 270K J. Boiling point C Decomposes before boiling. EFSA Scientific Publications 5 Verified data used for regulatory purposes-Degradation point C 355. It is a volatile organic compound and an alkane.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Research Triangle Park North Carolina. SiO2 550-600 Al2O3 40-70 B2O3 80-110 ZrO2 00-40 Na2O 95-135 K2O 00-40 CaO 10-50 MgO 00-20 Fe2O3 02 ZnO 20-50 BaO 30-60 F2 10. Calculate the conversion percentage of n-heptane. The data table below is an application guide and indicates the. 311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: sciencedirect.com
Source: sciencedirect.com
Thermodynam 1972 4 773-782. It is a volatile organic compound and an alkane. ILO International Chemical Safety Cards ICSC-050 C. There was no change in the crystal structure of the talc heated up to 800C. It is measured relative to a mixture of 224-trimethylpentane an octane and n- heptane.
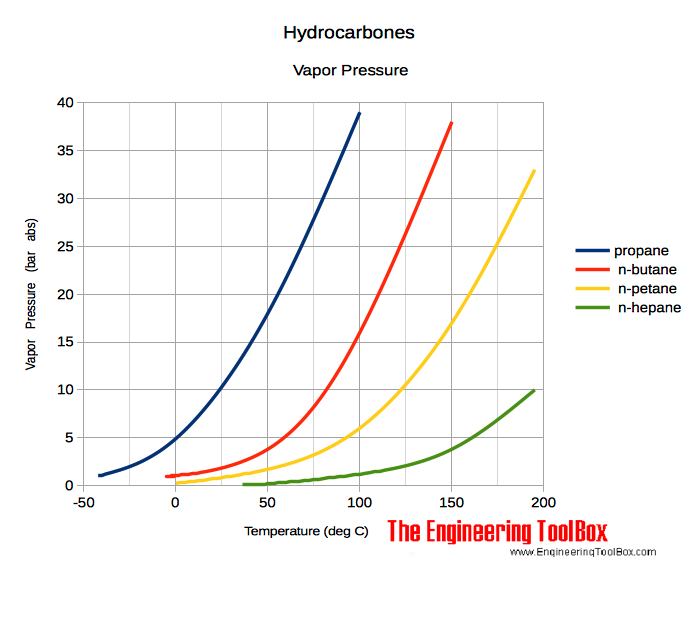 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Distinct solvent-like odour TGAI weak not characteristic pure AI Melting. EFSA Scientific Publications 5 Verified data used for regulatory purposes-Degradation point C 355. 10 is extracted with 250 mil of deionized water or reagent grade n-heptane at reflux temperature for 2 h. The data table below is an application guide and indicates the. Depending on the composition of the fuel the MON of a modern gasoline will be about 10 points.
 Source: spectrabase.com
Source: spectrabase.com
Carruth and Kobayashi 1973. It has been found in Jeffrey pine Pinus jeffreyi. 10 is extracted with 250 mil of deionized water or reagent grade n-heptane at reflux temperature for 2 h. Distinct solvent-like odour TGAI weak not characteristic pure AI Melting. EFSA Scientific Publications 5 Verified data used for regulatory purposes-Degradation point C 210.
 Source: researchgate.net
Source: researchgate.net
There was no change in the crystal structure of the talc heated up to 800C. So an 87-octane gasoline has the same knock resistance as a mixture of 87 isooctane and 13 n-heptane. At the temperature approximately 1200C the enstatite steadily. The research included the measurement of heat effects weight losses and changes in true specific gravity occurring on heating talc. 6 and that will be held on a US.
Source: webbook.nist.gov
Additional Application Specific n-Heptane. Why Consumption Of Lubricating Oil Is More In Two-stroke Cycle Petrol Engine Than Four-stroke Cycle Petrol Engine. Coefficents calculated by NIST from authors data. The data table below is an application guide and indicates the. 311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title weight and boiling point of n heptane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.