Vapor pressure of acetone at boiling point
Home » datasheet » Vapor pressure of acetone at boiling pointVapor pressure of acetone at boiling point
Vapor Pressure Of Acetone At Boiling Point. At the critical point there is no change of state when pressure is increased or if heat is added. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. Precipitation is water that falls from clouds in the skyIt may be rain liquid if it is warm or it may be frozen if it is cold. Acetone MSDS Page 2 of 2 Rev.
 Acetone Thermophysical Properties From engineeringtoolbox.com
Acetone Thermophysical Properties From engineeringtoolbox.com
Consider that the pressure above the liquid is pressing down on the surface making it difficult for the molecules to escape into the gas phase. The technique called acetone vapor bath smoothing involves placing the printed part in a sealed chamber containing a small amount of acetone and heating to around 80 degrees Celsius for 10 minutes. Acetone MSDS Page 2 of 2 Rev. This creates a vapor of acetone in the container. No known life can live without it. Lakes oceans seas and rivers are made of water.
It also shows the saturation pressure with changes in temperature.
And 2-propanone acetone which boils above the boiling point of either pure component. The more pressure the. Chlorpyrifos is an organic thiophosphate that is OO-diethyl hydrogen phosphorothioate in which the hydrogen of the hydroxy group has been replaced by a 356-trichloropyridin-2-yl group. Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. Surface tension then smooths the semi-liquid plastic. The technique called acetone vapor bath smoothing involves placing the printed part in a sealed chamber containing a small amount of acetone and heating to around 80 degrees Celsius for 10 minutes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. Chlorpyrifos is an organic thiophosphate that is OO-diethyl hydrogen phosphorothioate in which the hydrogen of the hydroxy group has been replaced by a 356-trichloropyridin-2-yl group. The technique called acetone vapor bath smoothing involves placing the printed part in a sealed chamber containing a small amount of acetone and heating to around 80 degrees Celsius for 10 minutes. Water - Saturation Pressure and Specific Weight - Vapor pressure and specific weight of water at temperatures ranging 32 to 212 o F - Imperial Units Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water. Boiling Point and Pressure.
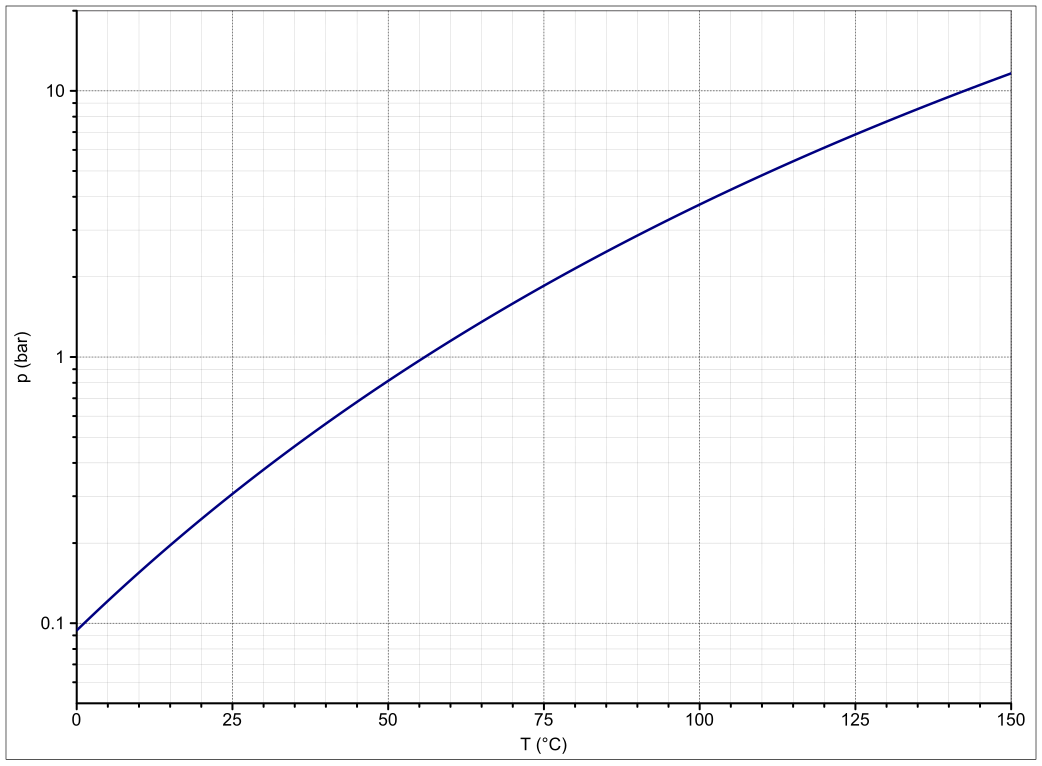 Source: commons.wikimedia.org
Source: commons.wikimedia.org
The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. No known life can live without it. At the normal boiling point of a liquid the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere. If water gets very cold below 0 C 32 F. Water H 2 O is a transparent tasteless odorless and almost colorless chemical substance and covers over 70 of Earths surface.
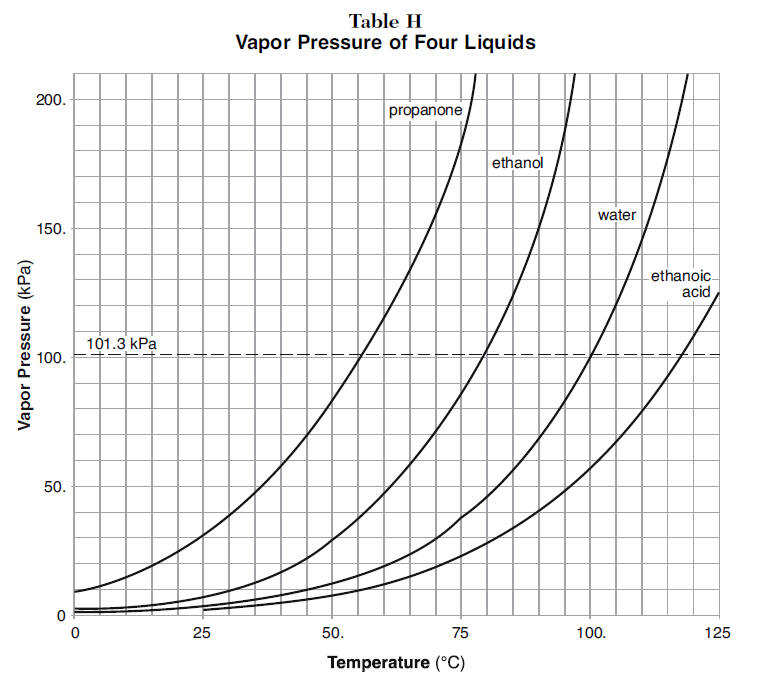 Source: kentchemistry.com
Source: kentchemistry.com
In our example the acetone in the solution had χ 064 but acetones mole fraction in the vapor equals 081. The vapor is richer in the component with the higher vapor pressure than the solution. No known life can live without it. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. The technique called acetone vapor bath smoothing involves placing the printed part in a sealed chamber containing a small amount of acetone and heating to around 80 degrees Celsius for 10 minutes.

The more pressure the. Surface tension then smooths the semi-liquid plastic. Water H 2 O is a transparent tasteless odorless and almost colorless chemical substance and covers over 70 of Earths surface. Boiling PointRange 56 C 1328 F Flash Point-20 C -4 F Method - CC. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Water - Saturation Pressure and Specific Weight - Vapor pressure and specific weight of water at temperatures ranging 32 to 212 o F - Imperial Units Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water. Surface tension then smooths the semi-liquid plastic. This creates a vapor of acetone in the container. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. Boiling Point and Dipole-Dipole Interactions.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The component with the higher vapor pressure. It has a role as an EC 3117 acetylcholinesterase inhibitor an agrochemical an EC 3118 cholinesterase inhibitor an environmental contaminant a xenobiotic an acaricide and an insecticide. The more pressure the. Boiling Point and Pressure. At the critical point there is no change of state when pressure is increased or if heat is added.

The technique called acetone vapor bath smoothing involves placing the printed part in a sealed chamber containing a small amount of acetone and heating to around 80 degrees Celsius for 10 minutes. Fire and Explosion Data Flash Point 14 Flammable Limits in Air By Volume. No known life can live without it. A key point about the above result is this. Boiling PointRange 56 C 1328 F Flash Point-20 C -4 F Method - CC.
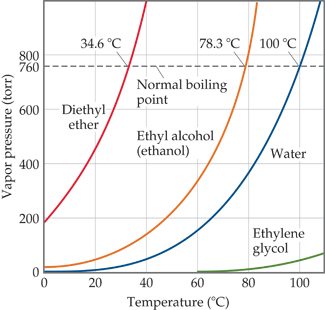 Source: clutchprep.com
Source: clutchprep.com
It has a role as an EC 3117 acetylcholinesterase inhibitor an agrochemical an EC 3118 cholinesterase inhibitor an environmental contaminant a xenobiotic an acaricide and an insecticide. It also shows the saturation pressure with changes in temperature. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. The component with the higher vapor pressure.
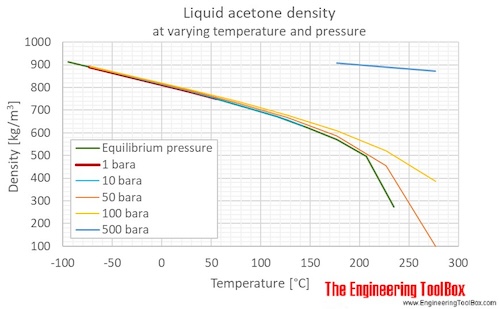 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Acetone Revision Date 25-Apr-2019 Vapor Pressure 247 mbar 20 C Vapor Density 20 Specific Gravity 0790 Solubility Soluble in water Partition coefficient. The vapor is richer in the component with the higher vapor pressure than the solution. It has a role as an EC 3117 acetylcholinesterase inhibitor an agrochemical an EC 3118 cholinesterase inhibitor an environmental contaminant a xenobiotic an acaricide and an insecticide. Acetone also can be used to extract fats oils. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure.
 Source: ddbst.com
Source: ddbst.com
Boiling Point and Dipole-Dipole Interactions. The component with the higher vapor pressure. The more pressure the. Surface tension then smooths the semi-liquid plastic. Acetone MSDS Page 2 of 2 Rev.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vapor pressure of acetone at boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.