Trimethylamine boiling point
Home » datasheet » Trimethylamine boiling pointTrimethylamine boiling point
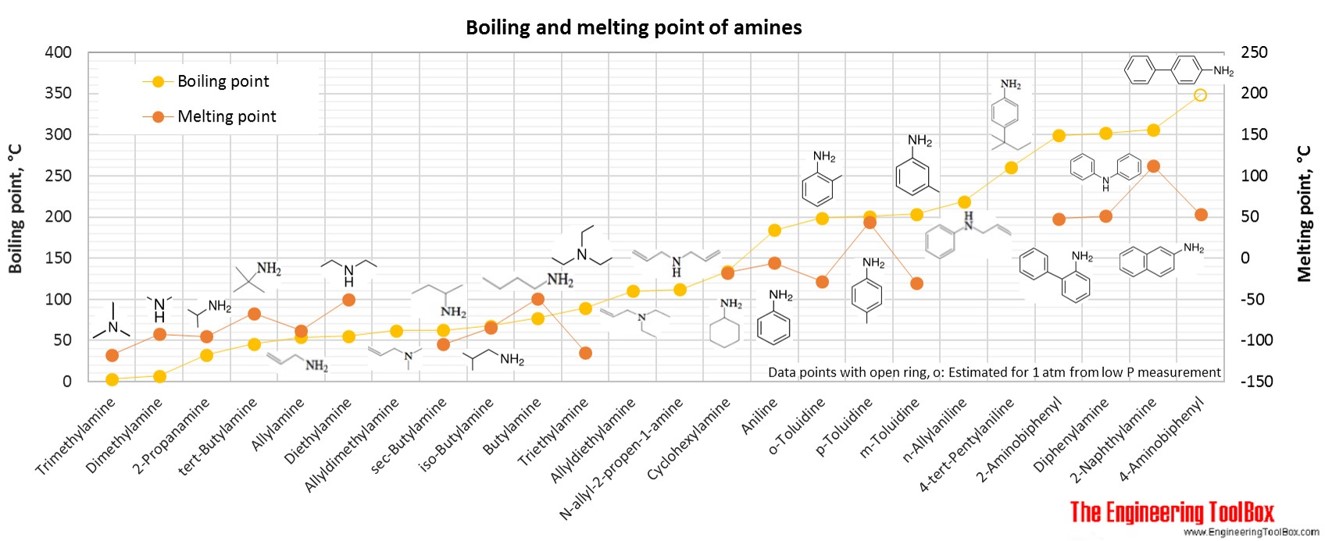
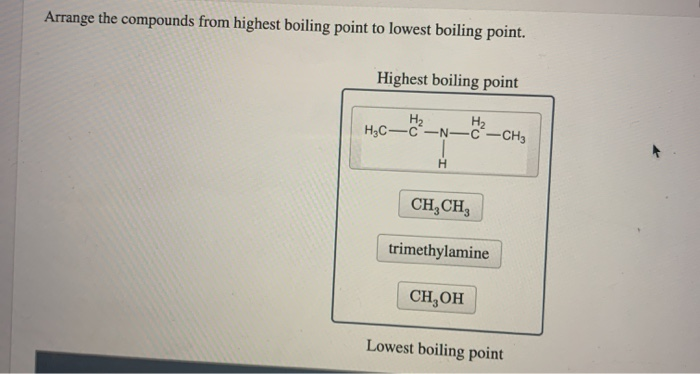
Trimethylamine Boiling Point. The lower boiling point is due to the lower dipole-dipole attractions in the. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. As one might readily guess the inclusion of a heteroatom such as nitrogen in otherwise exclusively carbon and hydrogen molecules has quite an effect on the properties of amines as compared to alkanes. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.

Carboxylic acid Alcohols 12 Amines 3 AminesAlkanes. As one might readily guess the inclusion of a heteroatom such as nitrogen in otherwise exclusively carbon and hydrogen molecules has quite an effect on the properties of amines as compared to alkanes. Alcohols boil considerably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. This Philippines most famous and iconic delicacy is prepared on special occasion and religious festivals but can also be found at street vendors as its a much sought dish by tourists. Extremely Hazardous Substances EHS Chemical Profiles and Emergency First Aid Guides.
3 to 7 C.
It is a tertiary amine and a member of methylamines. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. They have boiling points comparable to those of ethers Table 51 Physical Properties of Some Amines and. 37 to 44 F. The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Hazardous Substances Data Bank HSDB 18400to18500C760. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. In fact the melting and boiling points of oxygen are about -219C and -183C respectively. The molecular weight and boiling point of compounds are directly related with the flavor development eg esters and aldehyde have higher molecular weight but lower boiling point but lactones have relatively high boiling point. Carboxylic acid Alcohols 12 Amines 3 AminesAlkanes.

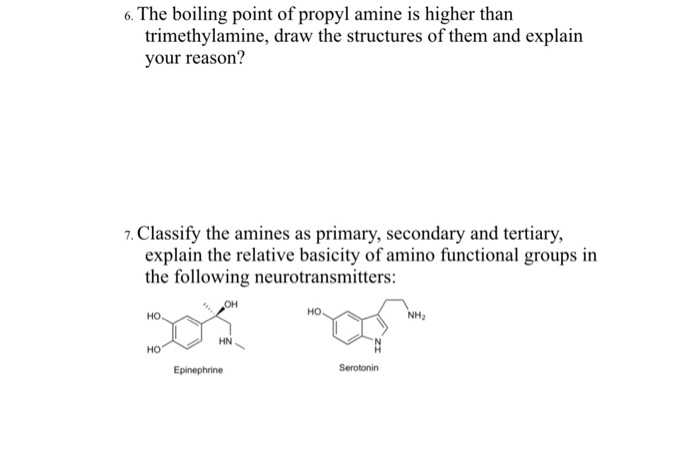
The ICSC project is a common undertaking between the World Health Organization WHO and. The main target users are workers and those responsible for occupational safety and health. Chapter 6 Amines and Amides 19 Physical Properties of Amines. H 2 NCH 2 CH 2 NH 2. Water Solubility 1 2 and 3 amines can all form.
 Source: chegg.com
Source: chegg.com
It is used in the synthesis of choline tetramethylammonium hydroxide plant growth regulators or herbicides. They have boiling points comparable to those of ethers Table 51 Physical Properties of Some Amines and. Three basic requirements must be met for explosion to take place. 1887 kPa at 20 C Henrys law. The boiling point is specific for the given substanceFor example the boiling point of.
 Source: chegg.com
Source: chegg.com
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Trimethylamine CH 3 3 N. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. Melting Point C Physical Form. Water Solubility 1 2 and 3 amines can all form.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Melting Point C Physical Form. It has a role as a human xenobiotic metabolite and an Escherichia coli metabolite. The primary aim of the cards is to promote the safe use of chemicals in the workplace. 363 to 367 F at 760 mm Hg EPA 1998 US. The lower boiling point is due to the lower dipole-dipole attractions in the.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Alcohols boil cosiderably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen. Alcohols boil cosiderably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen. Trimethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. TMA is widely used in industry. H 2 NCH 2 CH 2 NH 2.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Chapter 6 Amines and Amides 19 Physical Properties of Amines. CH 3 CO 2 H. For instance in the first example below the trisubstituted alkene is favoured over the mono-substituted alkene. Secondary amines still form hydrogen bonds but having the nitrogen atom in the middle of the chain rather than at the end makes the permanent dipole on the molecule slightly less. Source of ignition - spark or high heat.
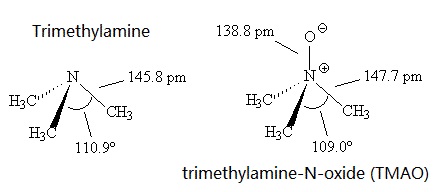 Source: chemicalbook.com
Source: chemicalbook.com
To eat Balut you are supposed to tap a hole on the top slurp. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms. H 2 NCH 2 CH 2 NH 2. HOCH 2 CH 2 OH. In a few cartilaginous fish like shark and skate cooking to 140F60C is needed to soften their connective tissue.
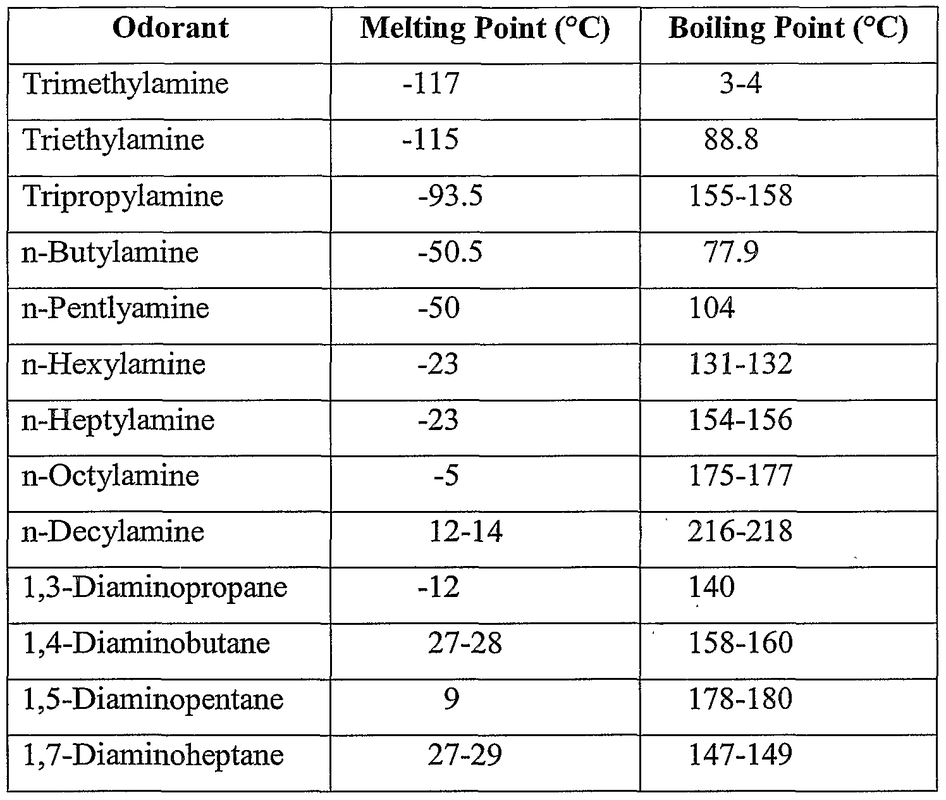 Source: organicchemistrystructures.weebly.com
Source: organicchemistrystructures.weebly.com
To eat Balut you are supposed to tap a hole on the top slurp. Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C. H 2 NCH 2 CH 2 NH 2. It is a tertiary amine and a member of methylamines. The boiling point of the secondary amine is a little lower than the corresponding primary amine with the same number of carbon atoms.
 Source: quizlet.com
Source: quizlet.com
Trimethylamine is a good nucleophile and this reaction is the basis of most of its applications. They have boiling points comparable to those of ethers Table 51 Physical Properties of Some Amines and. Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding. In fact the melting and boiling points of oxygen are about -219C and -183C respectively. The melting and boiling points of nitrogen are about.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title trimethylamine boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.