Triethanolamine melting point
Home » datasheet » Triethanolamine melting pointTriethanolamine melting point
Triethanolamine Melting Point. Exposure controls Engineering measures. Copies may be obtained from the American Society for Testing and. To C 59To F - 32 Unit Converter. Immediately flush eyes with running water for at least 15 minutes keeping eyelids open.
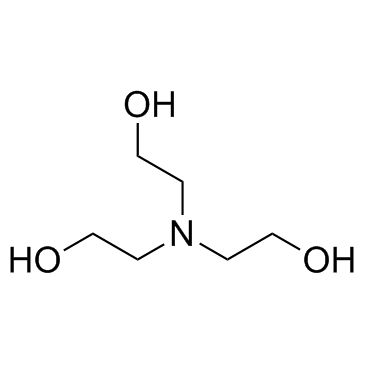 Triethanolamine Cas 102 71 6 Chemsrc From chemsrc.com
Triethanolamine Cas 102 71 6 Chemsrc From chemsrc.com
Cold water may be used. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Finish by rinsing thoroughly with. Exposure controls Engineering measures. It adjusts the melting point and thickens products. The number of surfactant molecules at the surface reaches a maximum ie.
Chemicals used to increase flexibility and strength of plastics and.
Personal protection equipment should be chosen according to relevant. Such stabilization is extremely important as it allows an increase. However isopropylbenzylamine is supposed to have a significantly HIGHER melting point so its odd that a It would be nice if there were tests there that didnt involve smoking it or injecting it. Exposure controls Engineering measures. 102-71-6 Triethanolamine - 5 mgm3 TWA Sen WES 111-46-6 22-Oxybisethanol - 23 ppm 100 mgm3 TWA - WES Notes. Above CMC - the surface tension remains constant.
 Source: en.wikipedia.org
Source: en.wikipedia.org
At the point where the surface becomes saturated micellization occurs in the bulk liquid. For my stomach it burned but I got used to it to the point where it didnt bother me and I seen results in my stretch marks that were deep red to a light pink on my stomach you would be surprised as to where my butt had some stretch marks and are so light now it makes my skin so smooth I love it. To F To C95 32. At the point where the surface becomes saturated micellization occurs in the bulk liquid. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more.
 Source: chemicalbook.com
Source: chemicalbook.com
To C 59To F - 32 Unit Converter. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Chemicals used to increase flexibility and strength of plastics and. So does meth take away life itself Like Heroin did before we got Cocaine on. It is a common ingredient in formulations used for both industrial and consumer products.
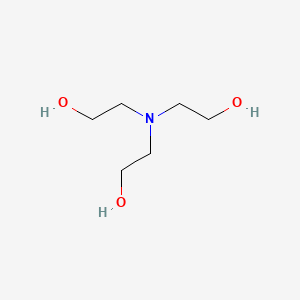
One possible strategy is to use high-melting-point materials such as Pd or Rh. It is a common ingredient in formulations used for both industrial and consumer products. Adsorption At SolidLiquid Interfaces Adsorption of surfactant from an aqueous solution onto a solid surface may involve specific chemical interaction between the. Chemicals used to increase flexibility and strength of plastics and. Toxicological Data on Ingredients.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It adjusts the melting point and thickens products. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. It adjusts the melting point and thickens products. 552a and 1 CFR part 51. To C 59To F - 32 Unit Converter.

So does meth take away life itself Like Heroin did before we got Cocaine on. Copies may be obtained from the American Society for Testing and. Cold water may be used. The triethanolamine neutralizes fatty acids adjusts and buffers the pH and solubilizes oils and other ingredients that are. Triethanolamine is used primarily in making surfactants such as for emulsifier.
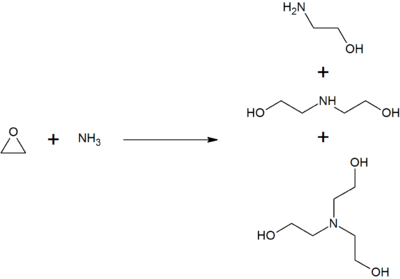 Source: en.wikipedia.org
Source: en.wikipedia.org
The number of surfactant molecules at the surface reaches a maximum ie. At the point where the surface becomes saturated micellization occurs in the bulk liquid. Observe occupational exposure limits and minimise the risk of inhalation of vapours. The number of surfactant molecules at the surface reaches a maximum ie. 102-71-6 Triethanolamine - 5 mgm3 TWA Sen WES 111-46-6 22-Oxybisethanol - 23 ppm 100 mgm3 TWA - WES Notes.

Adsorption At SolidLiquid Interfaces Adsorption of surfactant from an aqueous solution onto a solid surface may involve specific chemical interaction between the. Exposure controls Engineering measures. Only used it twice so far but I LOVE it. The copolymer shall have a melting point of 239 to 243 degC and a melt index of less than or equal to 20 as determined by ASTM Method D 3275-89 Standard Specification for E-CTFE-Fluoroplastic Molding Extrusion and Coating Materials which is incorporated by reference in accordance with 5 USC. It adjusts the melting point and thickens products.
 Source: guidechem.com
Source: guidechem.com
Immediately flush eyes with running water for at least 15 minutes keeping eyelids open. At the point where the surface becomes saturated micellization occurs in the bulk liquid. It is also abbreviated TEA yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium for which TEA is also a common abbreviation. Such stabilization is extremely important as it allows an increase. Immediately flush eyes with running water for at least 15 minutes keeping eyelids open.
 Source: chemsrc.com
Source: chemsrc.com
102-71-6 Triethanolamine - 5 mgm3 TWA Sen WES 111-46-6 22-Oxybisethanol - 23 ppm 100 mgm3 TWA - WES Notes. 102-71-6 Triethanolamine - 5 mgm3 TWA Sen WES 111-46-6 22-Oxybisethanol - 23 ppm 100 mgm3 TWA - WES Notes. Usually found in cleansers to dissolve oil and grease. To F To C95 32. Past incidents yes probably everyone who has used meth has had a bad dose at one time or another that was tainted but.
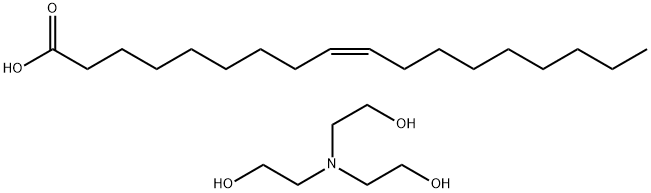 Source: chemicalbook.com
Source: chemicalbook.com
Toxicological Data on Ingredients. Such stabilization is extremely important as it allows an increase. To F To C95 32. Triethylamine is the chemical compound with the formula NCH 2 CH 3 3 commonly abbreviated Et 3 N. At the point where the surface becomes saturated micellization occurs in the bulk liquid.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title triethanolamine melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.