Toluene boiling point celsius
Home » datasheet » Toluene boiling point celsiusToluene boiling point celsius
Toluene Boiling Point Celsius. Academiaedu is a platform for academics to share research papers. 647 C 1485 F Boiling point of acetone. It can also be noted that formic acid forms. This weak acid is known to form a miscible mixture with water.
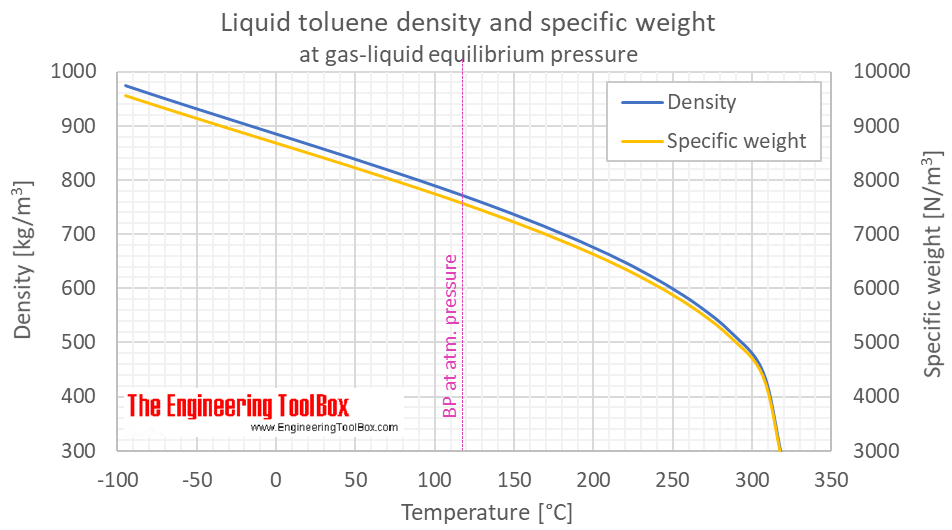 Toluene Density And Specific Weight Vs Teemperature And Pressure From engineeringtoolbox.com
Toluene Density And Specific Weight Vs Teemperature And Pressure From engineeringtoolbox.com
My guess is yes lowering the atmospheric pressure will only lower the boiling point it wont affect whether separation will occur. What pressure in atmospheres is exerted by 0120 mol of steam water vapor at its boiling point of 100degrees C if the volume is contained in a 200-L teakettle. Kf benzene 512 Cm 361 molal. Academiaedu is a platform for academics to share research papers. 647 C 1485 F Boiling point of acetone. When any liquid receives enough energy it starts to change its kinetic energy level and go from just a liquid to a liquid and some gas.
Figure 1311 Temperaturecomposition phase diagrams of binary systems with partially-miscible liquids exhibiting a the ability to be separated into pure components by fractional distillation b a minimum-boiling azeotrope and c boiling at a lower temperature than the boiling point of either pure component.
Kf benzene 512 Cm 361 molal. Water boils at a hundred degree Celsius and ethanol boils at around seventy-eight-degree Celsius whereas azeotropes boil at around seventy-eight-degree Celsius indicating that it has a lower boiling point than its constituents. A 10 m NaNO₃ B 10 m HF C 10 m C₆H₁₂O₆ D 10 m CaCl₂. Import numpy as np x nplinspace-nppi nppi 10 print x print x0 first element print x2 third element print x-1 last element print x-2 second to last element. It can either change into a gas when it has reached its boiling point or it evaporates which is just when surface molecules of a liquid get j. -1958 C -3204 F Boiling point of liquid helium.
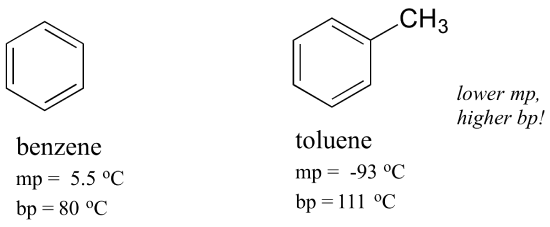 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
The conjugate base formed from the deprotonation of formic acid is commonly referred to as formate. The physical state gas liquid or solid depends on temperature and pressure but not on the mass of the substance. Boiling point of water. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Lauric acid or systematically dodecanoic acid is a saturated fatty acid with a 12-carbon atom chain thus having many properties of medium-chain fatty acids is a bright white powdery solid with a faint odor of bay oil or soapThe salts and esters of lauric acid are known as laurates. Carbon dioxide phase diagram. A 10 m NaNO₃ B 10 m HF C 10 m C₆H₁₂O₆ D 10 m CaCl₂. When any liquid receives enough energy it starts to change its kinetic energy level and go from just a liquid to a liquid and some gas. The boiling point of alcohols Oct 11 2005 Can a cyclohexane-toluene mixture be separated if the external pressure is 350mm HG instead of 760 mmHG.
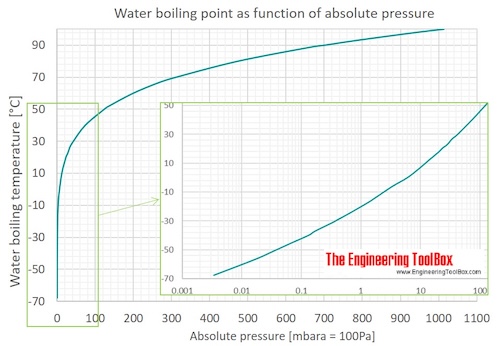 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point is specific for the given substanceFor example the boiling point of. Assuming that the variation of vapor pressure p with temperature T maybe described by the expression 𝑝 𝐵 log 𝐴 𝑘𝑃𝑎 𝑇 What are the values of A and B. MgCl₂ has a vant Hoff factor of 270.
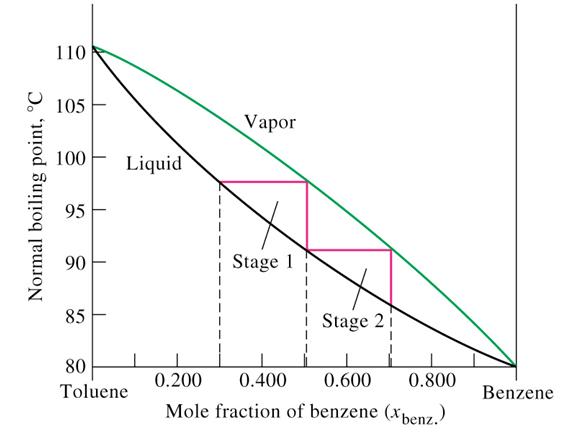 Source: web.inc.bme.hu
Source: web.inc.bme.hu
After this time the dispersion will gradually separatesediment out at a very slow rate resulting in lower concentrations and. My guess is yes lowering the atmospheric pressure will only lower the boiling point it wont affect whether separation will occur. The color of a substance does not change when you change the amount of substance. Kf benzene 512 Cm 361 molal. Lauric acid or systematically dodecanoic acid is a saturated fatty acid with a 12-carbon atom chain thus having many properties of medium-chain fatty acids is a bright white powdery solid with a faint odor of bay oil or soapThe salts and esters of lauric acid are known as laurates.
 Source: clutchprep.com
Source: clutchprep.com
What would be the boiling point. For example lets say that we have a solution made from two chemicals. 3732 K Boiling point of ethanol. 56 C 1328 F Boiling point of alcohol. Boiling point of a liquid is the temperature at which the vapour pressure of a liquid is equal to the external.
 Source: separationprocesses.com
Source: separationprocesses.com
60 mL of benzene and 60 of toluene. The color of a substance does not change when you change the amount of substance. HEATVAPZ HEATVAPZ_T HEATVAPZ_P HEATVALUE Heat of Formation Jmol MEA DEA 22-Iminodiethanol. The physical state gas liquid or solid depends on temperature and pressure but not on the mass of the substance. -269 C -452 F.

Academiaedu is a platform for academics to share research papers. Carbon dioxide CO 2 is a colourless and odorless gas. The boiling point is specific for the given substanceFor example the boiling point of. Unlike Matlab which uses parentheses to index a array we use brackets in python. 7837 C 1731 F Boiling point of nitrogen.
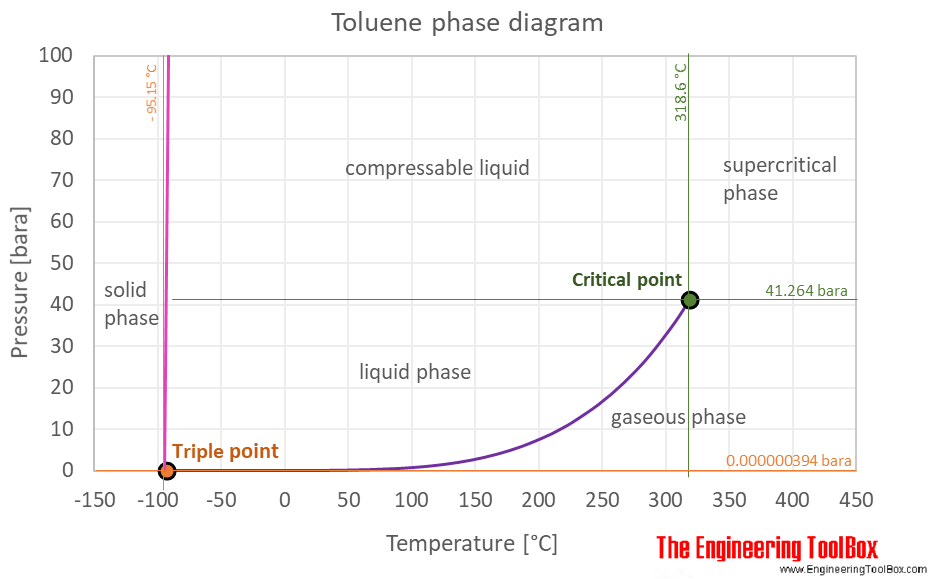 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
-269 C -452 F. 117 a Normal boiling point 360 K. It is relatively nontoxic and noncombustible but it is heavier than air and may asphyxiate by the displacement of air. 7837 C 1731 F Boiling point of nitrogen. In his paper Observations of two persistent degrees on a thermometer he recounted his experiments showing that the melting point of ice is essentially.
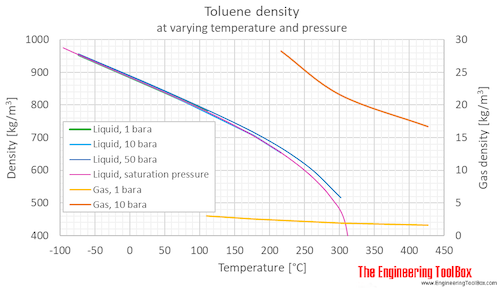 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Academiaedu is a platform for academics to share research papers. Assuming that the variation of vapor pressure p with temperature T maybe described by the expression 𝑝 𝐵 log 𝐴 𝑘𝑃𝑎 𝑇 What are the values of A and B. What would be the boiling point. 647 C 1485 F Boiling point of acetone. 60 mL of benzene and 60 of toluene.
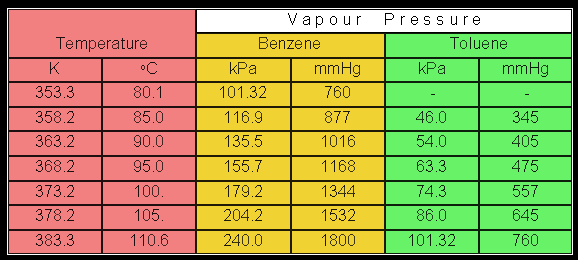 Source: separationprocesses.com
Source: separationprocesses.com
Boiling point of water. 116 The stronger the intermolecular forces the higher the boiling point of a liquid. Figure 1311 Temperaturecomposition phase diagrams of binary systems with partially-miscible liquids exhibiting a the ability to be separated into pure components by fractional distillation b a minimum-boiling azeotrope and c boiling at a lower temperature than the boiling point of either pure component. The melting point of formic acid is 84 degrees celsius whereas the boiling point of this compound corresponds to 1008 degrees celsius. MgCl₂ has a vant Hoff factor of 270.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title toluene boiling point celsius by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.