Thf boiling point
Home » datasheet » Thf boiling pointThf boiling point
Thf Boiling Point. The table above distinguishes between protic and aprotic solvents. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. 23 V air -190 C Upper Explosive Limit. Common Solvents Used in Organic Chemistry.
 Tetrahydrofuran American Chemical Society From acs.org
Tetrahydrofuran American Chemical Society From acs.org
Recent advances in the synthesis of these frameworks by reticulating predesigned SBUs and linker molecules confirm the potential of translating. The boiling point is an important property because it determines the speed of evaporation. Observe the position of the yellow point on eachMetals are materials most widely used in industry because of their properties. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph. The alkaline earth metals comprise the group 2 elements. Usually we mean things with a boiling point significantly higher than water.
The boiling point of pentane C 5 H 12 MW 72 is 36 C 97 F close to the.
C 4 H 10 O MW 74 is 118 C 244 F. One of the most susceptible solvents is diisopropyl ether but all ethers are considered to be potential peroxide sources. Reactions of Alkaline Earth Metals. Ethylene oxide is a flammable gas with a somewhat sweet odor. Place the container into a heat bath at about the same temperature and allow to cool slowly. The boiling point is an important property because it determines the speed of evaporation.
 Source: dalinyebo.com
Source: dalinyebo.com
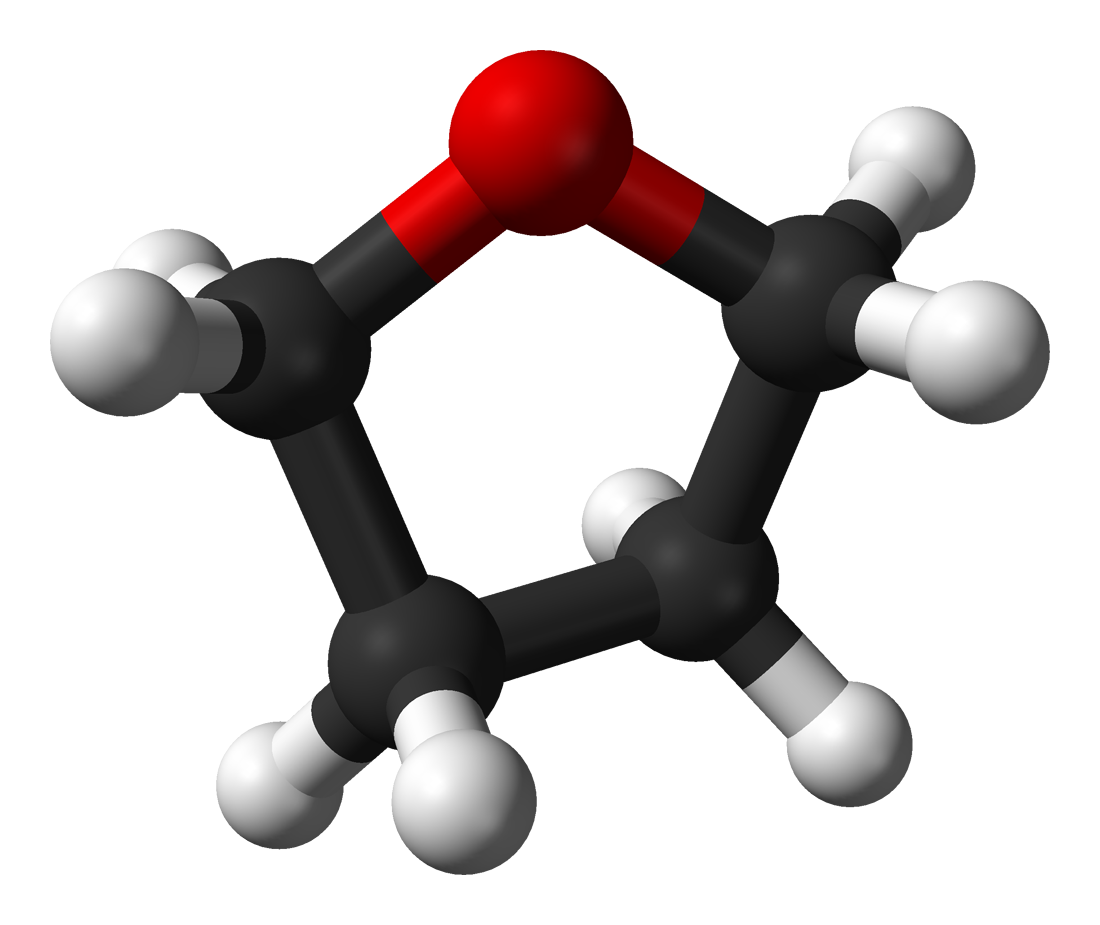
The compound is classified as heterocyclic compound specifically a cyclic ether. Physical and Chemical Properties Form. C 4 H 10 O MW 74 is 118 C 244 F. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. A variation of this method is to prepare a saturated solution at room.

Properties of Organic Solvents. The boiling point is an important property because it determines the speed of evaporation. Ethylene oxide is a flammable gas with a somewhat sweet odor. Transfer the solution into a clean container and cover. One of the most susceptible solvents is diisopropyl ether but all ethers are considered to be potential peroxide sources.
 Source: slideplayer.com
Source: slideplayer.com
C 2 H 4 O 2. The boiling point of pentane C 5 H 12 MW 72 is 36 C 97 F close to the. 103 C at 5 mm Hg. All the alkaline earth metals have two electrons in their valence shell so they lose two electrons to form cations with a 2 charge. Ethanol methanol tetrahydrofuran THF and acetone are usually not suitable for extraction because they are completely miscible with most aqueous solutions.

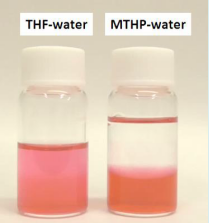
The alkaline earth metals comprise the group 2 elements. All the discovered alkaline earth metals occur in nature. One of the most susceptible solvents is diisopropyl ether but all ethers are considered to be potential peroxide sources. THF is useful in small-scale laboratory experiments mainly because it can dissolve a wide variety of organic compounds and has a relatively low boiling point making the solvent easy to remove. Information on the properties of common solvents used in organic chemistry including boiling points solubility density dielectric constants and flash points.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A Dewar with hot water frequently does the trick. 339 K Solubility in water. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. 1 mgcu m 034 ppm 1 ppm 295 mgcu m. 103 C at 5 mm Hg.
 Source: labchem-wako.fujifilm.com
Source: labchem-wako.fujifilm.com
It is a colorless water-miscible organic liquid with low viscosity. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. Recent advances in the synthesis of these frameworks by reticulating predesigned SBUs and linker molecules confirm the potential of translating. 1 mgcu m 034 ppm 1 ppm 295 mgcu m. For the solvents included in the table the distinguishing feature is the presence of an -OH group and that is the most common characteristic of a protic solvent.
 Source: ddbst.com
Source: ddbst.com
Hazardous Substances Data Bank HSDB Conversion factor. Reactions of Alkaline Earth Metals. C 4 H 10 O MW 74 is 118 C 244 F. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph.
 Source: sciencedirect.com
Source: sciencedirect.com
Oct 28 2011 The transition metals zinc Zn silver Ag and cadmium Cd form ions by losing one electron. One of the most susceptible solvents is diisopropyl ether but all ethers are considered to be potential peroxide sources. The alkaline earth metals comprise the group 2 elements. Small amounts of low-boiling-point solvents like. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
339 K Solubility in water. Usually we mean things with a boiling point significantly higher than water. One of the most susceptible solvents is diisopropyl ether but all ethers are considered to be potential peroxide sources. It is a colorless water-miscible organic liquid with low viscosity. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol.
 Source: acs.org
Source: acs.org
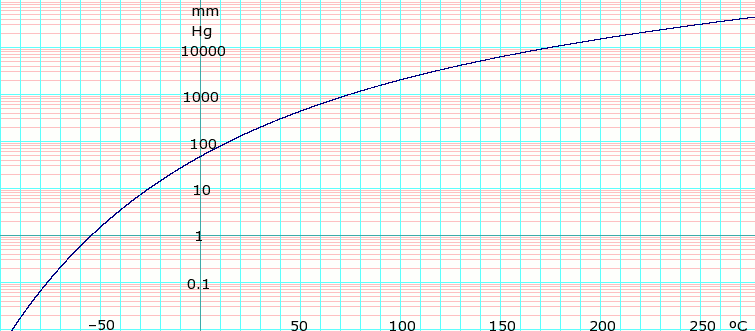
Boiling Temperature vs Chamber Pressure for Common Solvents-40-30-20-10 0 10 20 30 40 50 60 010 100 1000 10000. Small amounts of low-boiling-point solvents like. The heteroatom stabilizes the formation of a free radical. A variation of this method is to prepare a saturated solution at room. 23 V air -190 C Upper Explosive Limit.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title thf boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.