Tetrahydrofuran boiling point
Home » datasheet » Tetrahydrofuran boiling pointTetrahydrofuran boiling point
Tetrahydrofuran Boiling Point. Boiling point ºC Dielectric Constant Molecular Weight Acetic Acid-d 4 1165 1 204 5 22 17899 1 200 7 20 115 112 167 118 61 6408 Acetone-d 6 205 5 22 20668 1 2992 7 09 194 28 087 -94 565 207 6412 Acetonitrile-d 3 194 5 25 11869 1 139 7 21 21 084 -45 816 375 4407 Benzene-d 6 716 1 12839 3 243 04 095 55 801 23 8415 Chloroform-d. After two weeks of exposure the body burden of tetrahydrofuran seems to decrease. Ideally the selected solvents should be safe to use non-flammable and non-toxic in both liquid and vapor states. If water gets very cold below 0 C 32 F.

Waste disposal either by evaporation into the air or liquid disposal into wastewater should. 2-Methyltetrahydrofuran 2-MeTHF is an organic compound with the molecular formula C 5 H 10 O. No known life can live without it. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. The toy works at room temperature.
After a half-hour or so you can strain the plant.
Water H 2 O is a transparent tasteless odorless and almost colorless chemical substance and covers over 70 of Earths surface. It is a highly flammable mobile liquid. Other Information Links Identification CAS Number 104-80-3 Name 25-dihydroxymethyl tetrahydrofuran Synonyms 25-Anhydro-34-dideoxyhexitol ACDIUPAC Name 25-Anhydro-34-didesoxyhexitol German ACDIUPAC Name 25-Anhydro-34-didésoxyhexitol French ACDIUPAC Name 25-dihydroxymethyltetrahydrofuran 203-239-0 EINECS. Adult male rats exposed to tetrahydrofuran vapor at 82 200 ppm 41 1000 ppm or 82 mumoll 2000 ppm for 2 to 18 weeks five days a week 6 hrs daily showed dose-dependent brain and perirenal fat solvent burden linearly correlated to each other. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. Precipitation is water that falls from clouds in the skyIt may be rain liquid if it is warm or it may be frozen if it is cold.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. Common Boiling Dielectric Class Solvent Structure abbrevation pointC constant ePolarProticDonor hexane CH 3CH 2 4CH 3 68719 14-dioxane 1013 22 x benzene 80123 diethyl ether C 2H 5 2OEt 2O346 43 x chloroform CHCl 3 61248 ethyl acetate EtOAc 771 60 x acetic acid HOAc 1179 61 x x tetrahydrofuran THF 66 76 x methylene chloride CH 2Cl 2 39889 acetone. Boiling Point C Feature. 3 always be considered. Extremely Hazardous Substances EHS Chemical Profiles and Emergency First Aid Guides.
 Source: sciencedirect.com
Source: sciencedirect.com
The solvents should easily and. Other Information Links Identification CAS Number 104-80-3 Name 25-dihydroxymethyl tetrahydrofuran Synonyms 25-Anhydro-34-dideoxyhexitol ACDIUPAC Name 25-Anhydro-34-didesoxyhexitol German ACDIUPAC Name 25-Anhydro-34-didésoxyhexitol French ACDIUPAC Name 25-dihydroxymethyltetrahydrofuran 203-239-0 EINECS. If water gets very cold below 0 C 32 F. Boiling Point C Feature. Strain to Remove the Plant Material.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is also used as the fluid in Christmas bubble lights that have a colored bubbling tube above a lamp as a source of heat and a small amount of. If water gets very cold below 0 C 32 F. Extremely Hazardous Substances EHS Chemical Profiles and Emergency First Aid Guides. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. The toy works at room temperature.
 Source: acs.org
Source: acs.org
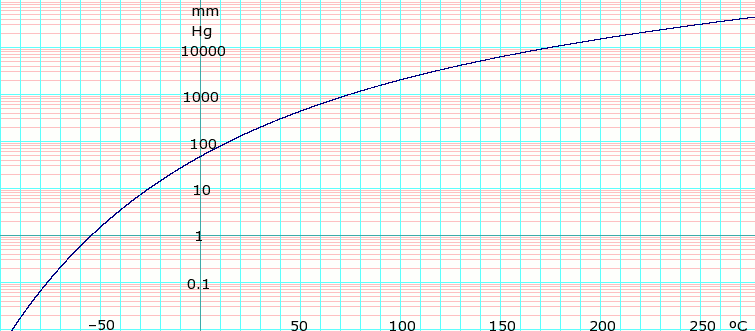
Ideally the selected solvents should be safe to use non-flammable and non-toxic in both liquid and vapor states. The low boiling point is convenient because it makes the solvent easy to remove from the chemical reaction by evaporation. Interestingly the boiling point of tetrahydrofuran C4H8O is 66 deg C because the ring gives it a permanent dipole moment. If water gets very cold below 0 C 32 F. This might have been caused by increased oxidative.
C 4 H 10 O MW 74 is 118 C 244 F. Extremely Hazardous Substances EHS Chemical Profiles and Emergency First Aid Guides. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Lakes oceans seas and rivers are made of water.
 Source: dalinyebo.com
Source: dalinyebo.com
3 always be considered. Strain to Remove the Plant Material. Lakes oceans seas and rivers are made of water. The chemical compounds low boiling point allows the chemical to function in a heat engine that can extract mechanical energy from small temperature differences. Iii NaOH dissolved in ethylene glycol and heated to near the boiling point.
 Source: ddbst.com
Source: ddbst.com
Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. After two weeks of exposure the body burden of tetrahydrofuran seems to decrease. Waste disposal either by evaporation into the air or liquid disposal into wastewater should. Commonly used solvents like ethyl acetate 81 diethyl ether 69 dichloromethane 13 and. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol.

Interestingly the boiling point of tetrahydrofuran C4H8O is 66 deg C because the ring gives it a permanent dipole moment. The selected solvents should have a relatively low boiling point to allow low temperature evaporation and leave no residue. An example of a DCM heat engine is the drinking bird. Hazardous Substances Data Bank HSDB 18400to18500C760. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More.

Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. The chemical compounds low boiling point allows the chemical to function in a heat engine that can extract mechanical energy from small temperature differences. Waste disposal either by evaporation into the air or liquid disposal into wastewater should. No known life can live without it. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights.
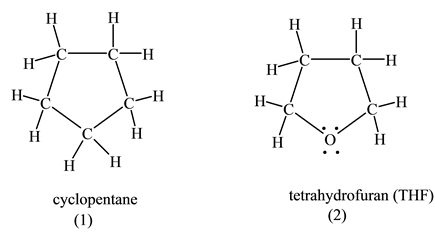 Source: chegg.com
Source: chegg.com
Strain to Remove the Plant Material. This might have been caused by increased oxidative. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. Common Boiling Dielectric Class Solvent Structure abbrevation pointC constant ePolarProticDonor hexane CH 3CH 2 4CH 3 68719 14-dioxane 1013 22 x benzene 80123 diethyl ether C 2H 5 2OEt 2O346 43 x chloroform CHCl 3 61248 ethyl acetate EtOAc 771 60 x acetic acid HOAc 1179 61 x x tetrahydrofuran THF 66 76 x methylene chloride CH 2Cl 2 39889 acetone. Melting Point C Physical Form.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title tetrahydrofuran boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.