T butylbenzene boiling point
Home » datasheet » T butylbenzene boiling pointT butylbenzene boiling point
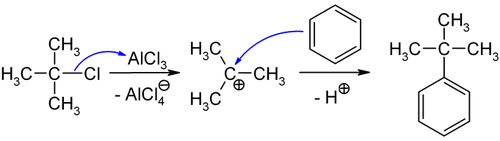
T Butylbenzene Boiling Point. 89 Y is the only stable isotope and the only. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. H4Si t-butylbenzene C10H14 toluene C7H8 trans-12-cis3-trimethylcyclopentane C8H16 trans-12-cis -4. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs.
Tert Butylbenzene Wikipedia From en.wikipedia.org
T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. H4Si t-butylbenzene C10H14 toluene C7H8 trans-12-cis3-trimethylcyclopentane C8H16 trans-12-cis -4. The transmission of polar effects through aromatic systems. Free Radicals Are Stabilized by Delocalization Resonance Secondly we have also learned that any factor which can lead to the electron deficient site being delocalized spread out over a larger area will also stabilize electron poor species. Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. This paper has data comparing the nitration of t-butylbenzene and toluene.
Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element.
89 Y is the only stable isotope and the only. 89 Y is the only stable isotope and the only. In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter. H4Si t-butylbenzene C10H14 toluene C7H8 trans-12-cis3-trimethylcyclopentane C8H16 trans-12-cis -4. The transmission of polar effects through aromatic systems. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a rare-earth element. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent. It is the fourteenth and penultimate element in the lanthanide series which is the basis of the relative stability of its 2 oxidation stateHowever like the other lanthanides its most common oxidation state is 3 as in its oxide halides and other compoundsIn aqueous solution like compounds of other late. Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element.
 Source: fishersci.pt
Source: fishersci.pt
This paper has data comparing the nitration of t-butylbenzene and toluene. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a rare-earth element. Yttrium is a chemical element with the symbol Y and atomic number 39. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a rare-earth element. The transmission of polar effects through aromatic systems. Previously for example weve seen that the positive charge of a carbocation is considerably stabilized when it is adjacent to a π bond. The nitration of benzyl derivatives J.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In order to compare the reactivity of donors two alcohols were used namely 2-propanol and 2-pentanol with the donor acceptor molar ratio DA equal to 6 or 8 and the catalytic tests were performed at the boiling point of the donor because it was used in large excess. The nitration of benzyl derivatives J. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a rare-earth element. H4Si t-butylbenzene C10H14 toluene C7H8 trans-12-cis3-trimethylcyclopentane C8H16 trans-12-cis -4. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs.
The transmission of polar effects through aromatic systems. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. It is the fourteenth and penultimate element in the lanthanide series which is the basis of the relative stability of its 2 oxidation stateHowever like the other lanthanides its most common oxidation state is 3 as in its oxide halides and other compoundsIn aqueous solution like compounds of other late. In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group.
Source: chemspider.com
It is the fourteenth and penultimate element in the lanthanide series which is the basis of the relative stability of its 2 oxidation stateHowever like the other lanthanides its most common oxidation state is 3 as in its oxide halides and other compoundsIn aqueous solution like compounds of other late. Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. The nitration of benzyl derivatives J. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs. In order to compare the reactivity of donors two alcohols were used namely 2-propanol and 2-pentanol with the donor acceptor molar ratio DA equal to 6 or 8 and the catalytic tests were performed at the boiling point of the donor because it was used in large excess.
Source: en.wikipedia.org
Ytterbium is a chemical element with the symbol Yb and atomic number 70. Yttrium is a chemical element with the symbol Y and atomic number 39. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent. Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter.
Source: chemspider.com
89 Y is the only stable isotope and the only. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group. 89 Y is the only stable isotope and the only. In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter. T-butylbenzene is much more p-directing than toluene 795 para for t-butylbenzene vs.
Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent. In the case of the former donor the reaction temperature was 355 K whereas it was 392 K for the latter. Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a rare-earth element. It is the fourteenth and penultimate element in the lanthanide series which is the basis of the relative stability of its 2 oxidation stateHowever like the other lanthanides its most common oxidation state is 3 as in its oxide halides and other compoundsIn aqueous solution like compounds of other late.
 Source: molinstincts.com
Source: molinstincts.com
89 Y is the only stable isotope and the only. Free Radicals Are Stabilized by Delocalization Resonance Secondly we have also learned that any factor which can lead to the electron deficient site being delocalized spread out over a larger area will also stabilize electron poor species. The nitration of benzyl derivatives J. Yttrium is a chemical element with the symbol Y and atomic number 39. 40 para for toluene which is likely due to sterics ortho approach is blocked by the bulkier t-butyl group.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title t butylbenzene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.