T butanol melting point
Home » datasheet » T butanol melting pointT butanol melting point
T Butanol Melting Point. Tert-Butyl hydroperoxide tBuOOH is the organic compound with the formula CH 3 3 COOH. Synthetic human hair wigs are made from a copolymer of vinyl chloride and. Alert regarding hydrogen balloon explosions. 1995S a Sn b Pb c Fe d Ag 39.

Amongst the following elements whose electronic configurations are. The t-butanol is a good solvent choice for this experiment. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. Alert regarding hydrogen balloon explosions. Its transition state from solid to liquid occurs near room temperature so it has a relatively low melting point thus a low freezing point. Chemical name of melamine is.
Therefore a 001m KF solution has a lower freezing point than a 001m solution of glucose.
Therefore a 001m KF solution has a lower freezing point than a 001m solution of glucose. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. Note that the potassium isnt so crucial here and we can leave it out or replace it with sodium or lithium- its really the t-butoxide part we really care about Potassium t-butoxide is like a really angry Sumo wrestler. If two materials have the same melting point then they may not necessarily have the same structure. Hydrogen Balloons Latex 1 Responses. 3 Chemical and Physical Properties Expand this section.
 Source: en.wikipedia.org
Source: en.wikipedia.org
New Window-137 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Therefore a 001m KF solution has a lower freezing point than a 001m solution of glucose. A Absolute molecular weight b Average molecular weight c Low molecular weight d Absolute melting point 15. It is normally supplied as a 6970 aqueous solution. We recently had an accident with a hydrogen balloon catching fire and melting rather than popping.
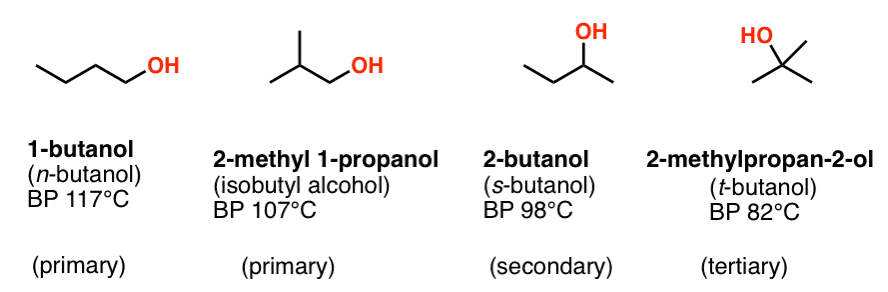 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Sign in to respond to this question. The molar mass of t-butanol 74123 gmol 1. Tert-Butyl alcohol is the simplest tertiary alcohol with a formula of CH 3 3 COH sometimes represented as t-BuOHIt is one of the four isomers of butanol. It is a hydroperoxide in fact one of the most widely used in a variety of oxidation processes for example the Halcon process. It also has a.
 Source: tcichemicals.com
Source: tcichemicals.com
Consider the isomers n-butanol and t-butanol. T F K F m. Forming the carbocation is the slow step. Which has most stable 2 oxidation state. New Window-137 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: iea-amf.org
Source: iea-amf.org
Experiment 1 post lab questions. Synthetic human hair wigs are made from a copolymer of vinyl chloride and. T F 01349. Melting points were measured on a Stuart Melting Point SMP3 apparatus Barloworld Scientific UK in open capillaries with uncorrected values. 1 H and 13 C-APT NMR spectra were recorded on a Bruker.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Forming the carbocation is the slow step. Experiment 1 post lab questions. Amongst H2O H2S H2Se and H2Te the one with the highest boiling point is 2000S a H2O because of hydrogen bonding b H2Te because of higher molecular weight c H2S because of hydrogen bonding d H2Se because of lower molecular weight 38. The molar mass of t-butanol 74123 gmol 1. 5 Related Records Expand this section.

Mass of solute10 g. New Window-137 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. 1 Structures Expand this section. National Toxicology Program Chemical Repository Database. 5 Related Records Expand this section.

4 Spectral Information Expand this section. National Toxicology Program Chemical Repository Database. It also has a. 1 Structures Expand this section. 2 Names and Identifiers Expand this section.
 Source: researchgate.net
Source: researchgate.net
USCG 1999 CAMEO Chemicals. From these freezing point measurements you will be able to calculate the molar mass of the unknown solute. Shows the list of common organic solvents their formula and melting point density solubility in water dielectric constant and flash point Pfizer solvent selection guide Red. It is a hydroperoxide in fact one of the most widely used in a variety of oxidation processes for example the Halcon process. The rate of the E1 reaction depends only on the substrate since the rate limiting step is the formation of a carbocationHence the more stable that carbocation is the faster the reaction will be.

T F K F m. FTIR-ATR spectra were recorded using a Fourier-Transform Infrared Attenuated Total Reflection UATR Two spectrometer PerkinElmer Waltham MA USA in the range 4504000 cm 1. Alert regarding hydrogen balloon explosions. Amongst H2O H2S H2Se and H2Te the one with the highest boiling point is 2000S a H2O because of hydrogen bonding b H2Te because of higher molecular weight c H2S because of hydrogen bonding d H2Se because of lower molecular weight 38. Amongst the following elements whose electronic configurations are.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16. Acid catalysed dehydration of t-butanol is faster than that of n-butanol because a tertiary carbocation is more stable than primary carbocation b primary carbocation is more stable than tertiary carbocation c t-butanol has a higher boiling point d rearrangement takes place during dehydration of t- butanol. 3 Chemical and Physical Properties Expand this section. Experiment 1 post lab questions. USCG 1999 CAMEO Chemicals.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title t butanol melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.