Solubility and boiling point
Home » datasheet » Solubility and boiling pointSolubility and boiling point
Solubility And Boiling Point. At its melting point the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. Our chief focus up to this point has been to discover and describe. Melting point - the temperature at which a solid turns into a liquid. 011 nm 2 Isotopes or mercury.
 Relationship Between Normal Boiling Point And Solubility For Candidate Download Scientific Diagram From researchgate.net
Relationship Between Normal Boiling Point And Solubility For Candidate Download Scientific Diagram From researchgate.net
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Boiling point of mercury. An Extensive Compilation of Aqueous Solubility Data for Organic Compounds Extracted from the AQUASOL dATAbASE. From equation 2 we get 2 o 7 3a b c 2 One can use Henrys law to calculate the amount of oxygen gas dissolved in water which is exposed to the atmosphere. Physical properties of matter include color hardness malleability solubility electrical conductivity density freezing points melting points and boiling points. The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break.
Therefore in this section the above.
The Henrys law constant for O gas in water at 25 C. Most molecular substances are insoluble or only very sparingly soluble in. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Dipole-dipole forces are not as strong as hydrogen bonds so dimethyl ether has a lower boiling point than methanol does. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. The boiling point of a solution was used to determine that santonic acid has a molecular mass of approximately 246.
 Source: researchgate.net
Source: researchgate.net
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website. The melting point is the temperature at which a solid changes into a liquid. Selection of method for solubility enhancement depends upon drug characteristics like solubility chemical nature melting point absorption site physical nature pharmacokinetic behavior and so forth dosage form requirement like tablet or capsule formulation strength immediate or modified release and so forth and regulatory requirements like maximum daily dose of any excipients andor. Although the terms mass and weight are used almost interchangeably there is a difference between themMass is a measure of the quantity of matter which is constant all over the universeWeight is proportional to mass but depends on location in the universeWeight is the force exerted on a body by gravitational attraction usually by the earth.
 Source: youtube.com
Source: youtube.com
Electronic shell of mercury Xe 4f 14 5d 10 6s 2. Is the temperature at which a liquid changes into a gas. Generally the measurement of the relative molecular mass boiling point and specific gravity of hydrocarbons are easy to obtain and the measurement accuracy is high. It will have the next highest boiling point. As with boiling points the melting point of a solid is dependent on the strength of those attractive forces.
 Source: slideserve.com
Source: slideserve.com
The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break. Boiling point of mercury. Boiling Point and Water Solubility. Melting point and freezing. From equation 2 we get 2 o 7 3a b c 2 One can use Henrys law to calculate the amount of oxygen gas dissolved in water which is exposed to the atmosphere.
 Source: breakingatom.com
Source: breakingatom.com
Examples of physical properties are temperature malleability appearance texture odour colour shape solubility melting freezing and boiling point. Melting point of mercury 389 C. Our chief focus up to this point has been to discover and describe. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F. Mixtures melt and boil over a range of temperatures.
 Source: quizlet.com
Source: quizlet.com
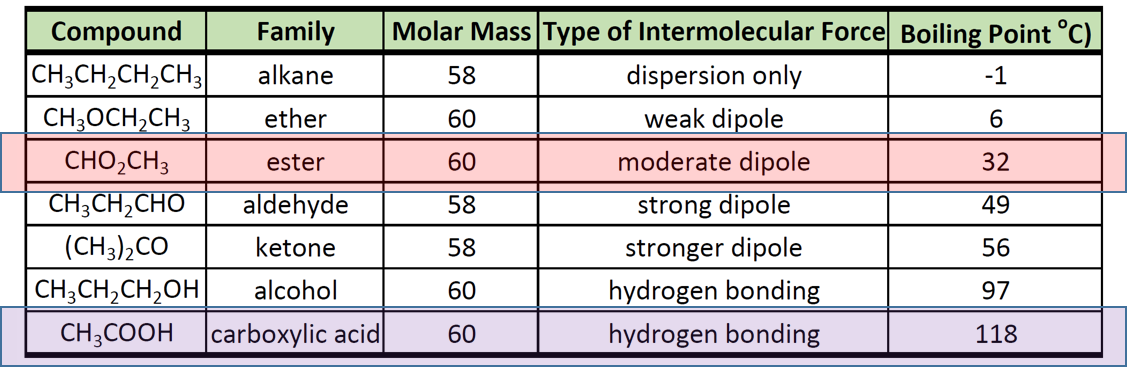
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. Corresponding -N-H—N- hydrogen. It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. Dimethyl ether CH_3OCH_3 is a polar molecule.
 Source: researchgate.net
Source: researchgate.net
The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. As with boiling points the melting point of a solid is dependent on the strength of those attractive forces. Dimethyl ether CH_3OCH_3 is a polar molecule. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Melting point and freezing.
 Source: youtube.com
Source: youtube.com
Corresponding -N-H—N- hydrogen. Boiling point - the temperature at which a liquid turns into a gas. Although the terms mass and weight are used almost interchangeably there is a difference between themMass is a measure of the quantity of matter which is constant all over the universeWeight is proportional to mass but depends on location in the universeWeight is the force exerted on a body by gravitational attraction usually by the earth. Boiling Point and Water Solubility. The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H—O- hydrogen bonding in alcohols light blue columns.
 Source: wou.edu
Source: wou.edu
An Extensive Compilation of Aqueous Solubility Data for Organic Compounds Extracted from the AQUASOL dATAbASE. Boiling point of mercury. The presence of hydrogen bonding will lift the melting and boiling points. The melting point is the temperature at which a solid changes into a liquid. Links with this icon indicate that you are leaving the CDC website.
 Source: chegg.com
Source: chegg.com
Melting point and freezing. Most molecular substances are insoluble or only very sparingly soluble in. Pure substances have specific melting and boiling points. Boiling point - the temperature at which a liquid turns into a gas. Selection of method for solubility enhancement depends upon drug characteristics like solubility chemical nature melting point absorption site physical nature pharmacokinetic behavior and so forth dosage form requirement like tablet or capsule formulation strength immediate or modified release and so forth and regulatory requirements like maximum daily dose of any excipients andor.

Sodium chloride NaCl is an ionic compound that consists of a multitude. Boiling and melting points hydrogen bonding phase diagrams polymorphism chocolate solubility. The Centers for Disease Control and Prevention CDC cannot attest to the accuracy of a non-federal website. Electronic shell of mercury Xe 4f 14 5d 10 6s 2. Our chief focus up to this point has been to discover and describe.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solubility and boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.