Sodium thiosulfate boiling point
Home » datasheet » Sodium thiosulfate boiling pointSodium thiosulfate boiling point
Sodium Thiosulfate Boiling Point. Chromic acid may also refer to the molecular species H 2 CrO 4 of which the trioxide is the anhydride. On burning will emit toxic fumes. The Only Reverse Osmosis Water Filter To Remove 99 Of Impurities. Production Chlorination of soda.
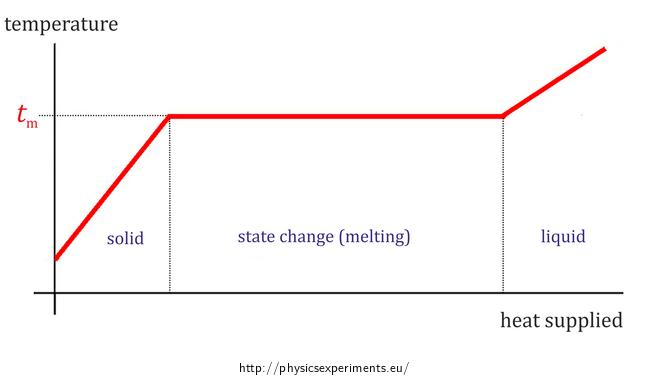 Melting Point Of Sodium Thiosulfate Pentahydrate Collection Of Experiments From physicsexperiments.eu
Melting Point Of Sodium Thiosulfate Pentahydrate Collection Of Experiments From physicsexperiments.eu
Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask. Melting Point of Potassium hydroxide. How many result combinations are there for the triple sugar-iron agar test when testing intestinal bacilli. Heparinized rat blood containing sodium 35S-sulfide was perfused through isolated rat lungs kidney or liver. Uses of Potassium Hydroxide. Investigate the effect of transition metal catalysts on the reaction between ironIII nitrate and sodium thiosulfate.
The precision of the end point varies with the sample.
The excess iodine is back-titrated with sodium thiosulfate or phenylarsine oxide. UOP555-10 Trace Impurities in Benzene by GC. Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask. Such use may cause explosion with release of toxic gases. SOLUTION OF SODIUM THIOSULFATE 01 moll In the volumetric lask. Illustrate the effect of a catalyst as sodium thiosulfate is oxidised by hydrogen peroxide in this demonstration.
 Source: study.com
Source: study.com
Academiaedu is a platform for academics to share research papers. Try this demonstration to. Heparinized rat blood containing sodium 35S-sulfide was perfused through isolated rat lungs kidney or liver. The precision of the end point varies with the sample. Academiaedu is a platform for academics to share research papers.
 Source: chemistrypage.in
Source: chemistrypage.in
Uses of Potassium Hydroxide. Heparinized rat blood containing sodium 35S-sulfide was perfused through isolated rat lungs kidney or liver. Molality moles of solutekg of water molliter. UOP563-14 Packed Apparent Bulk Density of Molecular Sieves. Prepare an emulsion of 5 g soluble starch in a mortar or beaker with a small amount of distilled water.
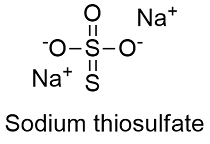 Source: softschools.com
Source: softschools.com
The Only Reverse Osmosis Water Filter To Remove 99 Of Impurities. In association with Nuffield Foundation. SOLUTION OF SODIUM THIOSULFATE 01 moll In the volumetric lask. An ounce of 1 sodium thiosulfate or milk of magnesia is helpful. Mass of solutetotal mass of solution100 molkg.
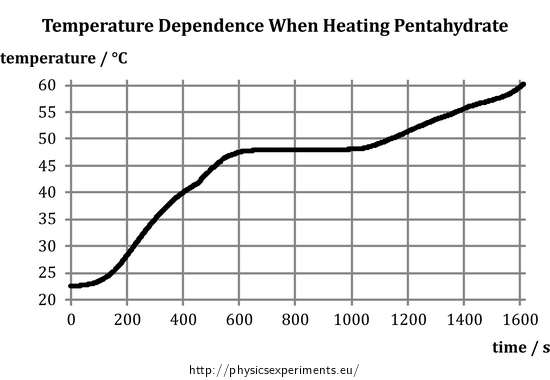 Source: physicsexperiments.eu
Source: physicsexperiments.eu
Sodium thiosulfate is an effective chlorine neutralizer. UOP563-14 Packed Apparent Bulk Density of Molecular Sieves. The precision of the end point varies with the sample. Catalysis of a sodium thiosulfate and ironIII nitrate reaction. Standard sodium thiosulfate titrant 00250N.
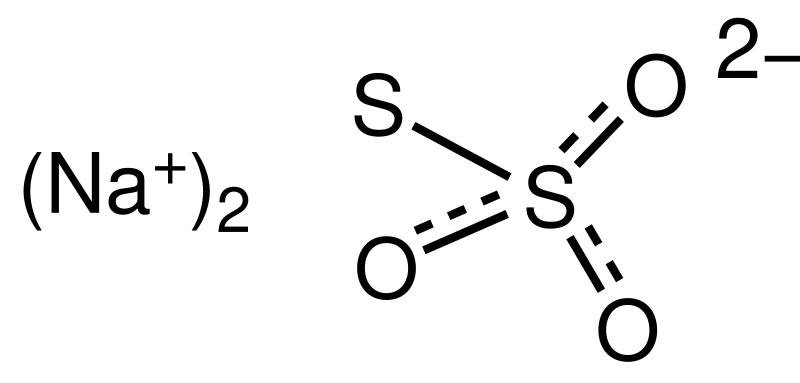 Source: en.wikipedia.org
Source: en.wikipedia.org
UOP551-08 C 6 and Lower Boiling Hydrocarbons in Olefin Free Naphthas by GC. It is used as a pH control agent in the food industry. In the TSI test sodium thiosulfate acts as a substrate for the measure of what reaction. UOP555-10 Trace Impurities in Benzene by GC. 1 choice of every household in Ireland 100 excellent reviews from our.

SOLUTION OF SODIUM THIOSULFATE 01 moll In the volumetric lask. Potassium hypochlorite was first produced in 1789 by Claude Louis Berthollet in his. SOLUTION OF SODIUM THIOSULFATE 01 moll In the volumetric lask. The Only Reverse Osmosis Water Filter To Remove 99 Of Impurities. The precision of the end point varies with the sample.
The low boiling point is convenient because it makes the solvent easy to remove from the chemical reaction by evaporation. Molarity moles of soluteliter of solution Values are tabulated below the figures. The rate and extent of sulfide oxidation varied from one organ to another. The low boiling point is convenient because it makes the solvent easy to remove from the chemical reaction by evaporation. UOP551-08 C 6 and Lower Boiling Hydrocarbons in Olefin Free Naphthas by GC.
 Source: physicsexperiments.eu
Source: physicsexperiments.eu
The Only Reverse Osmosis Water Filter To Remove 99 Of Impurities. Potassium hydroxide solution is more conductive when compared to NaOH and therefore used as an electrolyte in some alkaline batteries. The rate and extent of sulfide oxidation varied from one organ to another. Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask. On burning will emit toxic fumes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
UOP551-08 C 6 and Lower Boiling Hydrocarbons in Olefin Free Naphthas by GC. UOP549-09 Sodium in Petroleum Distillates by ICP-OES or AAS. Rinsing with a 5 mgL solution followed by washing with soap and water will remove chlorine odor from the hands. Molarity moles of soluteliter of solution Values are tabulated below the figures. In the isolated perfused lung system 35S-sulfide was oxidized slowly to 35S-thiosulfate.

1 choice of every household in Ireland 100 excellent reviews from our. 293 g100mL 0 C. Potassium hydroxide Structure KOH. How many result combinations are there for the triple sugar-iron agar test when testing intestinal bacilli. Heparinized rat blood containing sodium 35S-sulfide was perfused through isolated rat lungs kidney or liver.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium thiosulfate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.