Sodium sulfate boiling point
Home » datasheet » Sodium sulfate boiling pointSodium sulfate boiling point
Sodium Sulfate Boiling Point. Sodium Sulfate Anhydrous CAS-No. Identification Product form. Accessible as pure lustrous crystals granules or powder. 270 g100 mL water 20 C Solubility.
 Physical Properties Of Sodium Sulfate And Magnesium Sulfate Download Table From researchgate.net
Physical Properties Of Sodium Sulfate And Magnesium Sulfate Download Table From researchgate.net
As a result the LFR needs only two loops. Research Triangle Park North Carolina. Boiling point C 886 1413 De- composes Density at 20 C gcm3 071 217 253 Vapour pressure kPa 0133 Water solubility at 0 C gl reacts violently 357 71 infinitely soluble soluble Organoleptic properties The taste threshold for sodium in water depends on the associated anion and the temperature of the solution. Decomposes Solubility in water. With an annual production of 6 million tonnes the decahydrate is a major commodity chemical product. Try this class practical to make a plastic using potato starch and investigate the effects of adding a plasticiser.
Ozone is light blue.
Preparation of Aluminium Sulphate. Sodium sulfate also known as sodium sulphate or sulfate of soda is the inorganic compound with formula Na 2 SO 4 as well as several related hydratesAll forms are white solids that are highly soluble in water. Includes kit list and safety instructions. 270 g100 mL water 20 C Solubility. Includes kit list and safety instructions. 2575 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: trc-canada.com
Source: trc-canada.com
1 a primary loop with lead as a reactor coolant and 2 a secondary loop with watersteam as a Rankine power cycle. Making plastic from potato starch. As a result the LFR needs only two loops. It is mainly used as a filler in the manufacture of powdered home laundry. Decomposes Solubility in water.
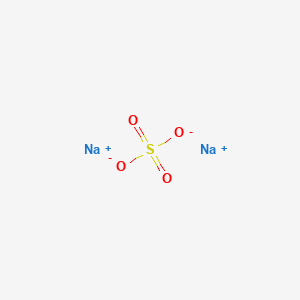
With an annual production of 6 million tonnes the decahydrate is a major commodity chemical product. Also it is a more inert liquid metal than sodium. 334 C 921 F. Sodium Sulfate Anhydrous CAS-No. Identification Product form.
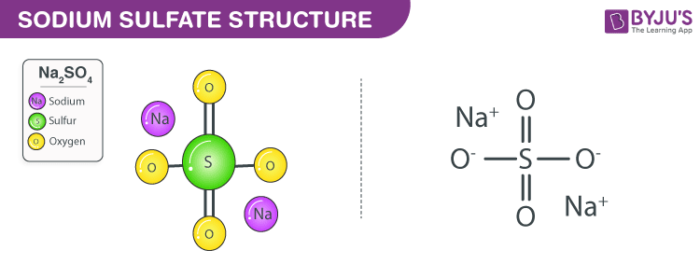 Source: byjus.com
Source: byjus.com
The solution that results is then evaporated and allowed to crystallize. National Toxicology Program Chemical Repository Database. Accessible as pure lustrous crystals granules or powder. 2575 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Includes kit list and safety instructions.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
8829 C 1621 F specific gravity. Its density is 1658 times that of air and it has a boiling point of 112 C 170 F at atmospheric pressure. As a result the LFR needs only two loops. 2 AlOH 3 3 H 2 SO 4 Al 2 SO 4 3 6 H 2 O. Properties and production.
 Source: en.wikipedia.org
Source: en.wikipedia.org
3065 K dehydration of heptahydrate 500 C anhydrous Boiling point. 11 04112018 EN English US Page 1 SECTION 1. Structure properties spectra suppliers and links for. Its density is 1658 times that of air and it has a boiling point of 112 C 170 F at atmospheric pressure. 334 C 921 F.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
Research Triangle Park North Carolina. The solution that results is then evaporated and allowed to crystallize. Its density is 1658 times that of air and it has a boiling point of 112 C 170 F at atmospheric pressure. 3065 K dehydration of heptahydrate 500 C anhydrous Boiling point. Properties and production.

2-8-1 or 1s 2 2s 2 2p 6 3s 1. Includes kit list and safety instructions. Preparation of Aluminium Sulphate. Lead has a higher melting point 3275C and significantly higher boiling point 1750C compared to that of sodium which significantly impacts on the reactor. Sodium Sulfate Anhydrous CAS-No.
 Source: researchgate.net
Source: researchgate.net
CRC Handbook of Chemistry and Physics. Also it is a more inert liquid metal than sodium. Preparation of Aluminium Sulphate. 1 a primary loop with lead as a reactor coolant and 2 a secondary loop with watersteam as a Rankine power cycle. 2575 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: studylib.net
Source: studylib.net
In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form applying electrolysis to fused. Because sodium is extremely reactive it never occurs in the free state in Earths crust. The solution that results is then evaporated and allowed to crystallize. 270 g100 mL water 20 C Solubility. 334 C 921 F.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Substance Substance name. As a result the LFR needs only two loops. In association with Nuffield Foundation. Accessible as pure lustrous crystals granules or powder. With an annual production of 6 million tonnes the decahydrate is a major commodity chemical product.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium sulfate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.