Sodium phosphate boiling point
Home » datasheet » Sodium phosphate boiling pointSodium phosphate boiling point
Sodium Phosphate Boiling Point. Aqueous solution is alkaline pH95. 10101-89-0 Sodium phosphate tribasic dodecahydrate USA Workplace Environmental Exposure Levels WEEL Appropriate Engineering controls. NA 0C Vapor Density AIR 1. For example water boils at 100C 212F at sea level but at 934C 2001.
 Material Safety Data Sheet Sodium Phosphate Dibasic From studylib.net
Material Safety Data Sheet Sodium Phosphate Dibasic From studylib.net
A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Boiling point of Sodium is 883C. Sodium fluoride reacts with chlorine undergoes displacement reaction forming sodium chloride and fluorine. Magnesium sulphate is reacted upon by caustic soda to form a precipitate of magnesium hydroxide. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. 10101-89-0 Sodium phosphate tribasic dodecahydrate USA Workplace Environmental Exposure Levels WEEL Appropriate Engineering controls.
Melting point 34-35 C when it contains the full 12 mols of H2O.
A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Sodium Melting Point and Boiling Point. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Soluble in 3 parts water. NaF Cl 2 NaCl F 2. Sodium fluoride has bactericidal properties but it is too toxic for use as a wound antiseptic.
Source:
Soluble in 3 parts water. Note that these points are associated with the standard atmospheric pressure. Boiling point of Sodium is 883C. For example water boils at 100C 212F at sea level but at 934C 2001. Hazardous Substances Data Bank HSDB.
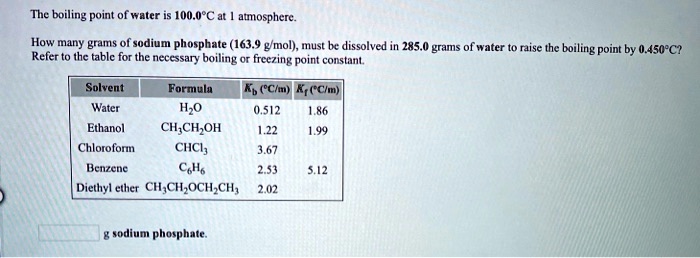 Source: numerade.com
Source: numerade.com
107 - 1093 Water 1. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Magnesium sulphate is reacted upon by caustic soda to form a precipitate of magnesium hydroxide. Sodium sulphate is highly soluble and remains in solution unless the water is. Sodium fluoride has bactericidal properties but it is too toxic for use as a wound antiseptic.
Source: chemspider.com
In general boiling is a phase change of a substance from the liquid to the gas phase. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. Sodium Phosphate Monobasic 10049-21-5 NA NA Sodium Phosphate Dibasic 7558-79-4 NA NA Boiling Point approx. Melting point - the temperature at which a solid turns into a liquid. Dihydrogen sodium phosphate anhydrous Recommended Use Laboratory chemicals.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Sodium fluoride has bactericidal properties but it is too toxic for use as a wound antiseptic. It is recommended that all dust control equipment such as local exhaust. In general boiling is a phase change of a substance from the liquid to the gas phase. Calcium Phosphate Solubility Ca3PO42. Trisodium Phosphate Created by Global Safety Management Inc.
 Source: studylib.net
Source: studylib.net
NA 0C Vapor Density AIR 1. In general boiling is a phase change of a substance from the liquid to the gas phase. Trisodium Phosphate Created by Global Safety Management Inc. 060103 Page 1 of 4 125 SODIUM HYPOCHLORITE SOLUTION Material Safety Data Sheet Emergency 24 Hour Telephone. NaF Cl 2 NaCl F 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Note that these points are associated with the standard atmospheric pressure. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. Solubility is one of the most important characteristics of calcium phosphate salts. Boiling Point Melting Point Critical Temperature Specific Gravity Vapor Pressure Vapor Density Volatility.
Source:
23 kPa 20C. Sodium Melting Point and Boiling Point. Dihydrogen sodium phosphate anhydrous Recommended Use Laboratory chemicals. Uses advised against Food drug pesticide or biocidal product use. Aqueous solutions of reducing sugars other than sucrose when heated above 84C evolve toxic levels of carbon monoxide in the presence of alkalis or alkaline salts such as sodium phosphate also potassium hydroxide sodium hydroxide calcium hydroxide etc Bretherick 5th ed.
 Source: studylib.net
Source: studylib.net
Uppercase M is molarity which is moles of solute per liter of solution not solvent. Aqueous solution is alkaline pH95. Melting point - the temperature at which a solid turns into a liquid. The item of commerce is often partially hydrated and may range from anhydrous Na 3 PO 4 to the. Note that these points are associated with the standard atmospheric pressure.
Source:
Merck and Co Inc 2006 p. Sodium Phosphate Monobasic 10049-21-5 NA NA Sodium Phosphate Dibasic 7558-79-4 NA NA Boiling Point approx. 125 SODIUM HYPOCHLORITE SOLUTION HASA 125 Sodium Hypochlorite Solution Material Safety Data Sheet MSDS No. In general boiling is a phase change of a substance from the liquid to the gas phase. Solubility is one of the most important characteristics of calcium phosphate salts.
 Source: studylib.net
Source: studylib.net
TSP is used as a cleaning agent builder lubricant food additive stain remover and degreaser. Hot andor concentrated NaOH can cause hydroquinone to decompose exothermically at elevated temperature. The item of commerce is often partially hydrated and may range from anhydrous Na 3 PO 4 to the. Irritant Safety data sheet. Sodium fluoride has bactericidal properties but it is too toxic for use as a wound antiseptic.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium phosphate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.