Sodium nitrite boiling point
Home » datasheet » Sodium nitrite boiling pointSodium nitrite boiling point
Sodium Nitrite Boiling Point. With Hach Service Plans you have a global partner who understands your needs and cares about delivering timely high-quality service you can trust. Do not allow to boil dry. As a liquid boiling point minus 1958C it is also colorless and odorless and is similar in appearance to water. It is on the World Health Organizations List of Essential Medicines.
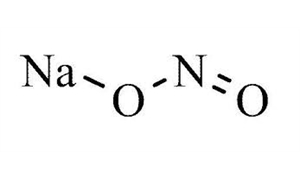 7632 00 0 Cas Sodium Nitrite Inorganic Salts Article No 05954 From lobachemie.com
7632 00 0 Cas Sodium Nitrite Inorganic Salts Article No 05954 From lobachemie.com
Common types of foods that are boiled include. Nitrogen as a gas is colorless odorless and generally considered an inert element. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Chemical Properties of Sodium Oxide Na 2 O. Physical Properties of Sodium Oxide Na 2 O. This compound has a sweet odour.
Sodium nitrate is a crystalline solid which is white.
Note that boiling water does not remove nitrates and is not a treatment alternative. Sediment debris and lead particles can collect in your aerator. Sort by Relevance. Sodium nitrate is a crystalline solid which is white. At C10 level the elements present will be told to you but read up the method. It is not necessary to use the sodium thiosulfate component of the.

This compound is also highly soluble in ammonia. With Hach Service Plans you have a global partner who understands your needs and cares about delivering timely high-quality service you can trust. Boiling Point C Feature. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. 100 o for 30 minutes more time at high altitude.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Analytical 4 ACS reagent 2 BioReagent 2 Technique. Note that boiling water does not remove nitrates and is not a treatment alternative. Place the basin on the trivetsaucer and add boiling water to come 13 of the way up the basin. Food additive and preservative. Melting Point C Physical Form.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Determine the boiling point or melting point. Sodium oxide reacts with carbon dioxide to form. Discontinue use of amyl nitrite when. Adult Until sodium nitrite becomes available break one ampule of amyl nitrite into a cloth. Nitrite reacts with the meat myoglobin to cause color changes first converting to.
 Source: chemistrylearner.com
Source: chemistrylearner.com
380 o C 653 K Decomposes 2. It is on the World Health Organizations List of Essential Medicines. Place in a steamer over boiling water or alternatively use a large saucepan with a trivet or an upturned heat-resistant saucer. Note that boiling water does not remove nitrates and is not a treatment alternative. Intravenous injections and intratracheal instillation of 13N-labelled nitrite in mice and rabbits resulted in homogenous.
 Source: sciencemadness.org
Source: sciencemadness.org
This compound is also highly soluble in ammonia. Food additive and preservative. The taste threshold for sodium in water depends on the associated anion and the temperature of the solution. Hach Service Protect your investment and peace of mind. Chemical Properties of Sodium Oxide Na 2 O.
 Source: coolgyan.org
Source: coolgyan.org
For example when each sodium atom in a sample of sodium metal group 1 gives up one electron to form a sodium cation Na and each chlorine atom in a sample of chlorine gas group 17 accepts one electron to form a chloride anion Cl the resulting compound NaCl is composed of sodium ions and chloride ions in the ratio of one Na ion for each Cl ion. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. A new ampule of amyl nitrite should be broken into a cloth every 3 minutes. Kills everything except some endospores. Boiling point - the temperature at which a liquid turns into a gas.
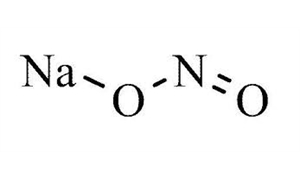 Source: lobachemie.com
Source: lobachemie.com
Nitrogen gas can be prepared by heating a water solution of ammonium nitrite NH 4 NO 3. For example when each sodium atom in a sample of sodium metal group 1 gives up one electron to form a sodium cation Na and each chlorine atom in a sample of chlorine gas group 17 accepts one electron to form a chloride anion Cl the resulting compound NaCl is composed of sodium ions and chloride ions in the ratio of one Na ion for each Cl ion. Sodium nitrate is a white deliquescent solid very soluble in water. Adult Until sodium nitrite becomes available break one ampule of amyl nitrite into a cloth. It serves the dual purpose of determining the bp as well as purification of the liquid for subsequent tests.
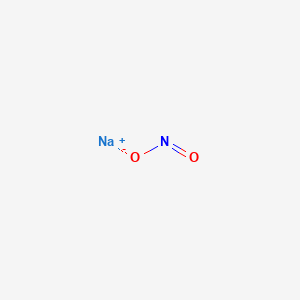
Nitrite is normally absent from the body fluids and tissues of laboratory animals. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. 100 o for 30 minutes more time at high altitude. 30 seconds every minute until an intravenous line is established followed by intravenous sodium nitrite 300 mg over absolutely no less than 5 minutes. It is not necessary to use the sodium thiosulfate component of the.
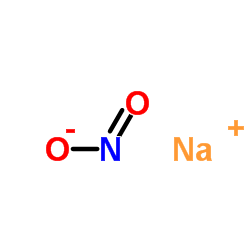 Source: chemsrc.com
Source: chemsrc.com
Melting Point C Physical Form. Dilute potassium solutions to taste. REG 10 ppm 0001 sodium nitrite - Alone as color fixative in smoked cured tuna -172175. Increasing the concentration leads to a bitter or alkaline flavor. Kills everything except some endospores.
 Source: echemi.com
Source: echemi.com
Boiling is the cooking of foods in a liquid eg water milk or stock which is at boiling point. -77F -603C Vapor pressure. Boiling Point C Feature. Nitrogen gas can be prepared by heating a water solution of ammonium nitrite NH 4 NO 3. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium nitrite boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.