Sodium methoxide boiling point
Home » datasheet » Sodium methoxide boiling pointSodium methoxide boiling point
Sodium Methoxide Boiling Point. Joint FAOWHO Expert Committee on Food Additives JECFA Colorless liquid with a fruity odor. 300 to 313 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Thus the boiling point of amines is higher than those for the corresponding phosphines compounds containing phosphorus but generally lower than the corresponding alcohols. A solution of sodium ethoxide is prepared from 60 g.
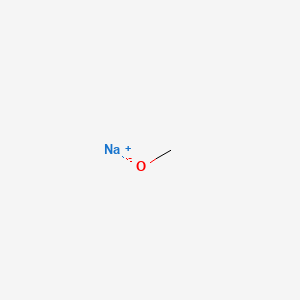
Sodium Methoxide Ch3nao Chemspider From chemspider.com
To the reaction mixture was added 20 sodium methoxide in methanol 43 g at 55 C. 15168 Phenyl-2-Propanone from Benzyl Cyanide. ILO International Chemical Safety Cards ICSC Colourless liquid with a sharp rum-like odour. Melting Point C Physical Form. Solid sodium methoxide which is insoluble in the reaction mixture was used as a basic homogeneous catalyst. Sodium 1-amino-4-2-methyl-5-4-methylphenylsulfonylaminophenylaminoanthraquinone-2-sulfonate 400-100-8 84057-97-6 016-066-00-9 tetrasodium 5-4-amino-6-chloro-135-triazin-2-ylamino-2-2-hydroxy-35-disulfonatophenylazo-2- sulfonatobenzylidenehydrazinobenzoatecopperII 404-070-7 116912-62-0 016-067-00-4 4-methylphenylmesitylene sulfonate 407-530-5 67811-06-7 016-068-00-X.
A high reboiler duty was required for MeOH and PGME separation in C1 because of the high fraction of the low boiling point component at the top.
2 Na 2 CH 3 OH 2 CH 3 ONa H 2. Sort by Relevance. Analytical 4 ACS reagent 2 BioReagent 2 Technique. National Toxicology Program Chemical Repository Database. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. The resulting solution which is colorless is often used as a source of sodium methoxide but the pure material can be isolated by evaporation followed by heating to remove residual methanol.
Gml 20C Refractive Index. Sodium bisulfate NaHSO 4 15 is then added to neutralize the mixture. Sort by Relevance. A solution of sodium ethoxide is prepared from 60 g. Wash with ice water ethanol then ether all cold to yield 16 g of product melting at 145-147.
The reaction mixture was stirred at reflux for 3 hours. Sodium methoxide is added 17 g and the mixture is stirred for 10 min at -10 to -12. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Mixing of alcohol and catalyst The catalyst is typically sodium hydroxide caustic soda or potassium hydroxide potash. 2 Na 2 CH 3 OH 2 CH 3 ONa H 2.
 Source: chemistrylearner.com
Source: chemistrylearner.com
ILO International Chemical Safety Cards ICSC Colourless liquid with a sharp rum-like odour. Illustrated Glossary of Organic Chemistry A product of the Institute for Reduction of Cognitive Entropy in Organic Chemistry. Typically 05 M NaOCH 3 in anhydrous methanol is added to the lipid extract and the solution is reacted at 45 C for 5 min. Therefore improving the energy efficiency of these distillation columns should inevitably bring great benefits to the entire. The reaction mixture was stirred at reflux for 3 hours.
 Source: chemyq.com
Source: chemyq.com
The reaction mixture was stirred at reflux for 3 hours. Less dense than water. Ch3o formal charge. To the hot solution added a mixture of 234g. The SN2 Reaction Mechanism.
Source: chemspider.com
15168 Phenyl-2-Propanone from Benzyl Cyanide. Analytical 4 ACS reagent 2 BioReagent 2 Technique. The suspension was filtered to obtain wet crystals 433 g of sodium salt of dimethyl 4-oxothiolane-23. Melting Point C Physical Form. Its called the S N 2 reaction and its going to be extremely useful for us going forward.
 Source: sdylhgtrade.com
Source: sdylhgtrade.com
Analytical 4 ACS reagent 2 BioReagent 2 Technique. The high boiling point of sodium allows the reactor to operate at ambient normal pressure but the drawbacks include its opacity which hinders visual maintenance and its explosive properties. A solution of sodium ethoxide is prepared from 60 g. Boiling Point C Feature. To the hot solution added a mixture of 234g.
 Source: degruyter.com
Source: degruyter.com
Less dense than water. Sodium methoxide is prepared by treating methanol with sodium. Wash with ice water ethanol then ether all cold to yield 16 g of product melting at 145-147. The radioactivity stops within a few days after removal from the reactor. Sodium bisulfate NaHSO 4 15 is then added to neutralize the mixture.
 Source: gelest.com
Source: gelest.com
To the hot solution added a mixture of 234g. Gml 20C Refractive Index. 300 to 313 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Vapors heavier than air. Under a nitrogen atmosphere.
Source: chem.nlm.nih.gov
Sodium methoxide is prepared by treating methanol with sodium. Wash with ice water ethanol then ether all cold to yield 16 g of product melting at 145-147. Melting Point C Physical Form. The radioactivity stops within a few days after removal from the reactor. Sort by Relevance.
 Source: fishersci.com
Source: fishersci.com
The resulting solution which is colorless is often used as a source of sodium methoxide but the pure material can be isolated by evaporation followed by heating to remove residual methanol. The reaction is so exothermic that ignition is possible. A solution of sodium ethoxide is prepared from 60 g. 26 mol clean sodium and 700 ml of absolute alcohol dried over calcium oxide or sodium in a 2000 ml round-bottomed flask equipped with a reflux condenser. Radioactive sodium-24 may be produced by neutron bombardment during operation posing a slight radiation hazard.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium methoxide boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.