Sodium hydroxide in methanol melting point
Home » datasheet » Sodium hydroxide in methanol melting pointSodium hydroxide in methanol melting point
Sodium Hydroxide In Methanol Melting Point. To recrystallize the naphthalene heat about 50 mL of the 31 methanolwater mixture almost to the boiling point on a steam bath see p. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14.
 Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table From researchgate.net
Effect Of Sodium Hydroxide And Potassium Hydroxide In Methanol On The Download Table From researchgate.net
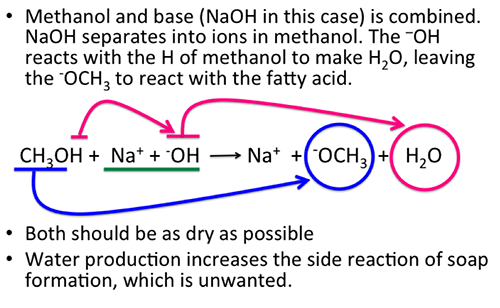
Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. A large amount of heat is released during the dissolution of solid sodium. Then add dilute ammonium hydroxide dropwise until the precipitate just dissolves. Slightly hazardous in case of inhalation lung sensitizer. This only works with anhydrous sodium hydroxide because combined with water the fat would turn into soap which would be tainted with methanol. However this can change if very concentrated solutions are used see table in the back of the reader.
Research Triangle Park North Carolina.
Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Polyvinyl alcohol has a melting point of 180 to 190C. Add 10 mL of a 1 M sodium hydroxide solution to the funnel. 596 K Boiling point. Boiling Point C Feature. The process of hydrolysis is based on.
Source: chemspider.com
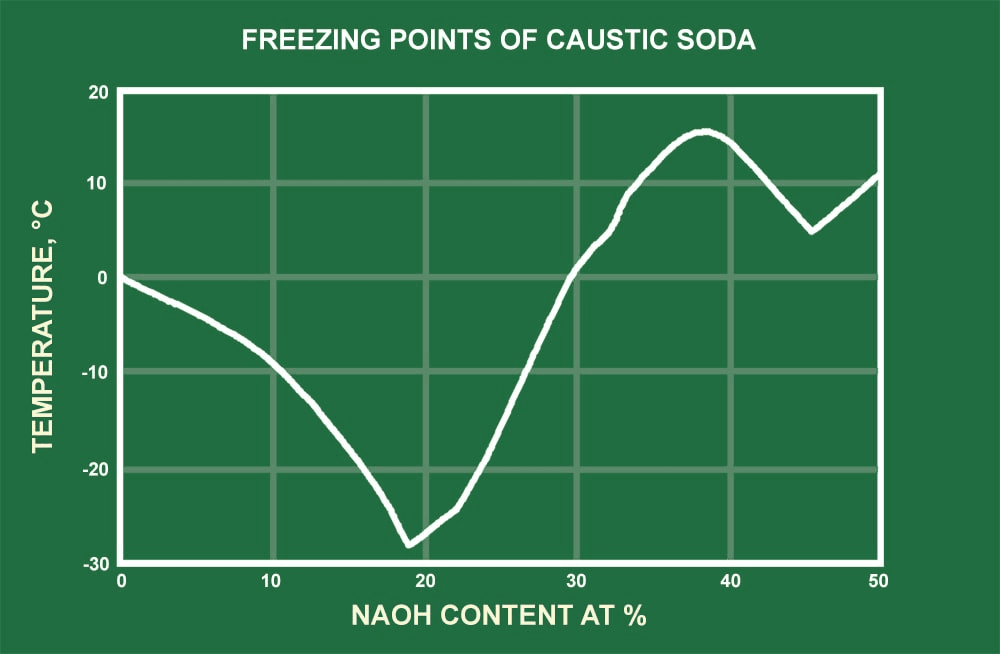
Allow the layers to separate completely remove the stopper from the funnel and then draw off the lower layer into a labeled 50 mL Erlenmeyer flask. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Polyvinyl alcohol has a melting point of 180 to 190C. It will dissolve in a hot mixture of 31 methanolwater but is less soluble when this mixture is cold. However this can change if very concentrated solutions are used see table in the back of the reader.

Polyvinyl alcohol has a melting point of 180 to 190C. At the melting point the solid and liquid phases exist in equilibrium. Hazardous in case of skin contact corrosive of eye contact corrosive. The health hazards of potassium hydroxide are similar to those of the other strong alkalies such as sodium. KOH CO 2 KHCO 3.
When dissolving solid NaOH in water or a strong acid. The addition of hydroxide ions by adding lime sodium hydroxide or potassium hydroxide adjusts the pH because the hydroxide ion reacts with carbon dioxide to form bicarbonate alkalinity. Boiling Point C Feature. The melting point is also referred to as liquefaction point solidus or liquidus. A large amount of heat is released during the dissolution of solid sodium.
 Source: fishersci.com
Source: fishersci.com
It is manufactured by the polymerization of vinyl acetate followed by partial hydrolysis. CRC Handbook of Chemistry and Physics. At the melting point the solid and liquid phases exist in equilibrium. It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. Liquid or spray mist may.
 Source: e-education.psu.edu
Source: e-education.psu.edu
The melting point is specific for a given substance. Slightly hazardous in case of inhalation lung sensitizer. Preparation of the reagent. To 1 mL of silver nitrate solution add a few drops of sodium hydroxide. The molecular weight of sodium hydroxide is 40 gmol.
 Source: researchgate.net
Source: researchgate.net
For example the melting point of ice frozen water is 0 C. A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. KOH CO 2 KHCO 3. Add 2 - 3 drops of the compound in methanol to 2 - 3 mL of Tollens solution contained in a very clean test tube. Solid NaOH has a melting point and boiling point of approximately 604F and 2534F.
Source:
We say that such a body melts. Crystalline and colourless it is solid. For the manufacture of biodiesel sodium hydroxide is used as a catalyst for the transesterification of methanol and triglycerides. The molecular weight of sodium hydroxide is 40 gmol. 1474 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
 Source: chemistrypage.in
Source: chemistrypage.in
The process of hydrolysis is based on. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Sodium methylate is a white amorphous powder. The health hazards of potassium hydroxide are similar to those of the other strong alkalies such as sodium. Add some of this hot solvent to.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Health Hazards of KOH. It is also a temperature at which a solid crystal turns into a liquid. We say that such a body melts. 323 C 613 F. The solvent is highly dispersible in water but less so in cold solvents such as methanol and ethanol.
 Source: protank.com
Source: protank.com
The health hazards of potassium hydroxide are similar to those of the other strong alkalies such as sodium. The melting point depends on the pressure. Sodium hydroxide is also the most common base used in chemical laboratories. Naphthalene is soluble in methanol and insoluble in water. Worldwide production in 1998 was around 45 million tonnes.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium hydroxide in methanol melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.