Sodium citrate boiling point
Home » datasheet » Sodium citrate boiling pointSodium citrate boiling point
Sodium Citrate Boiling Point. Analytical 4 ACS reagent 2 BioReagent 2 Technique. It is a soft bright silvery metal that floats on water. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. Magnesium is an essential element in both plant and animal life.
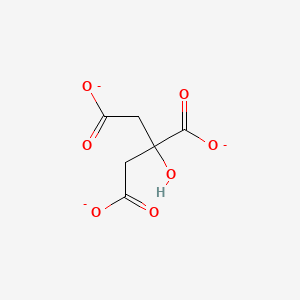
Molarity moles of soluteliter of solution Values are tabulated below the figures. Add 05 mL Tween 20 and mix well. The substances are listed in alphabetical order. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. Molality moles of solutekg of water molliter. Uses Of Sulfuric Acid.
See also density of aqueous solutions of inorganic chlorides inorganic potassium salts some other inorganic substances organic acids and organic.
Uses Of Oxalic Acid. Chlorophyll is the chemical that allows plants to. Adjust pH to 60 with 1N HCl. Adjust to pH 80 with NaOH. Analytical 4 ACS reagent 2 BioReagent 2 Technique. Its most common compound is sodium chloride table salt but.
 Source: yumpu.com
Source: yumpu.com
The substances are listed in alphabetical order. In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. 1090C 1994F 1363 K Block. Distilled water 1 L. Sodium is never found free in nature.
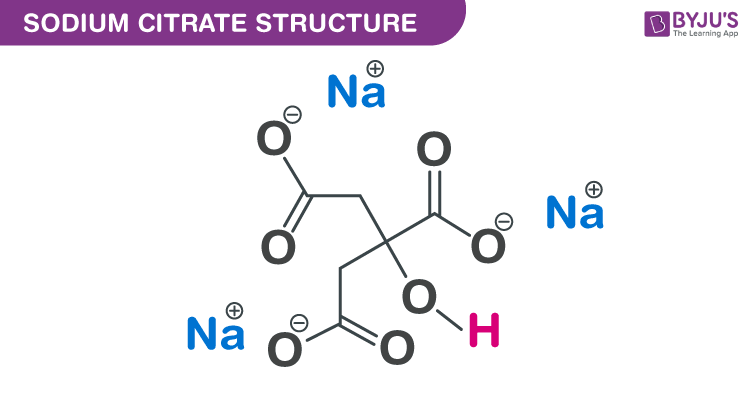 Source: byjus.com
Source: byjus.com
Adjust pH to 60 with 1N HCl. Uses Of Sodium Hydroxide. It is a soft bright silvery metal that floats on water. The substances are listed in alphabetical order. Grignard reagents are organic magnesium compounds that are important for the chemical industry.
Source: chemspider.com
Adjust pH to 60 with 1N HCl. Piperazine is a cyclic organic compound possessing two nitrogen atoms in opposite positions within a 6-member heterocyclic ring that serves as a backbone for piperazine derivatives and acts as a gamma-amino-butyric acid receptor agonist in nematodes with potential anti-helminthic activityUpon administration piperazine binds to the GABA inhibitory receptors in susceptible nematodes thereby. Magnesium is a chemical element with the symbol Mg and atomic number 12. Sodium citrate buffer 10 mM Sodium citrate 005 Tween 20 pH 60 Tri-sodium citrate dihydrate 294 g. Store at room temperature for 3.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Uses Of Oxalic Acid. Sodium is fairly abundant in the sun and stars the fourth most abundant element on Earth and the most commonly found alkali metal. 1 mM EDTA pH 80. Be aware of the concentration units in the figures. Boiling Point C Feature.
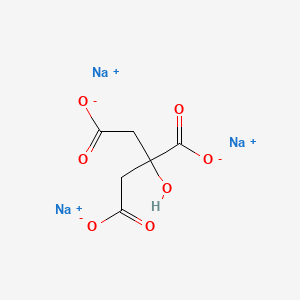
Magnesium is a chemical element with the symbol Mg and atomic number 12. Uses Of Oxalic Acid. See also density of aqueous solutions of inorganic chlorides inorganic potassium salts some other inorganic substances organic acids and organic. Distilled water 1 L. Grignard reagents are organic magnesium compounds that are important for the chemical industry.
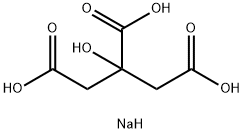 Source: chemicalbook.com
Source: chemicalbook.com
Magnesium is an essential element in both plant and animal life. Solutions with varied molarities have different properties ie a low molarity acid and high molarity acid can. Chlorophyll is the chemical that allows plants to. In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. Molarity moles of soluteliter of solution Values are tabulated below the figures.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. Sodium is never found free in nature. Boiling Point C Feature. Store at room temperature for 3. Sort by Relevance.
 Source: chemsrc.com
Source: chemsrc.com
All group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. Uses Of Ascorbic Acid. Molality moles of solutekg of water molliter. It is a soft bright silvery metal that floats on water. 1 mM EDTA pH 80.
 Source: molecularrecipes.com
Source: molecularrecipes.com
Uses Of Oxalic Acid. Magnesium is an essential element in both plant and animal life. Uses Of Sodium Hydroxide. Store at room temperature for 3 months or at. Uses Of Sulfuric Acid.

Molarity is the concentration of x moles of solute in 1 L of solution. Piperazine is a cyclic organic compound possessing two nitrogen atoms in opposite positions within a 6-member heterocyclic ring that serves as a backbone for piperazine derivatives and acts as a gamma-amino-butyric acid receptor agonist in nematodes with potential anti-helminthic activityUpon administration piperazine binds to the GABA inhibitory receptors in susceptible nematodes thereby. 1090C 1994F 1363 K Block. Solutions with varied molarities have different properties ie a low molarity acid and high molarity acid can. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium citrate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.