Sodium carbonate solution boiling point
Home » datasheet » Sodium carbonate solution boiling pointSodium carbonate solution boiling point
Sodium Carbonate Solution Boiling Point. Slight Bleach Bulk Density. Their composition was debated by early chemists and the solution finally came from the Royal. Sodium Chloride CAS. At sea level atmospheric pressure is high and water.
 Sodium Carbonate Wikipedia From en.wikipedia.org
Sodium Carbonate Wikipedia From en.wikipedia.org
The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Na 2 CO 3 2HCl 2NaCl CO 2 H 2 O. High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. Its melting point is 18. The general method of ester preparation can be. Boiling point - the temperature at which a liquid turns into a gas.
Its boiling point is 101.
High temperatures in the boiler water reduce the solubility of calcium sulphate and tend to make it precipitate out directly on the boiler metal as scale. When dissolving solid NaOH in water or a strong acid. 1661 K Solubility in water. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Lye-water is an essential ingredient in the crust of the traditional baked Chinese moon cakes. 0 360oC thermometers.
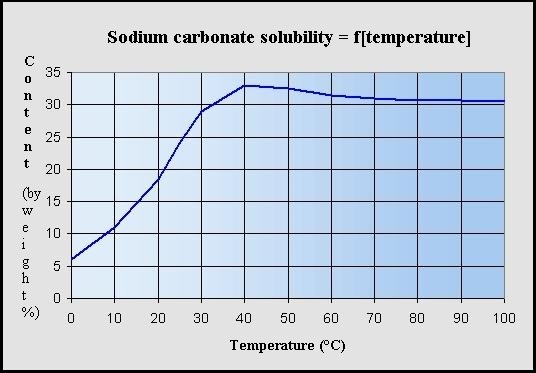 Source: hydro-land.com
Source: hydro-land.com
National Toxicology Program Chemical Repository Database. Saturated sodium carbonate solution 1 Bottle. October 26 2009 Sodium Bisulfite Solution Page 3 of 4 Respirators. Melting point - the temperature at which a solid turns into a liquid. While weighing do not spill the substance on.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Aqueous solutions of sodium carbonate are mildly alkaline due to hydrolysis which releases OH aq ions. Boiling Point 122C 252F Phosphoric Acid. Sodium Chloride CAS. W1 1325 g. 1388 C 2530 F.
 Source: researchgate.net
Source: researchgate.net
Boiling point - the temperature at which a liquid turns into a gas. 120 at 20ºC 68ºF Odor. Gloves The Synthesis of Ethyl Ethanoate. Sodium Carbonate CAS. Be aware of the concentration units in the figures.
It is soluble in. Because sodium is extremely reactive it never occurs in the free state in Earths crust. It melts without decomposition at 852 o C. Since calcium carbonate is relatively insoluble it tends to come out of solution. When titrated with hydrochloric acid carbonate decomposes yielding carbon dioxide and water.
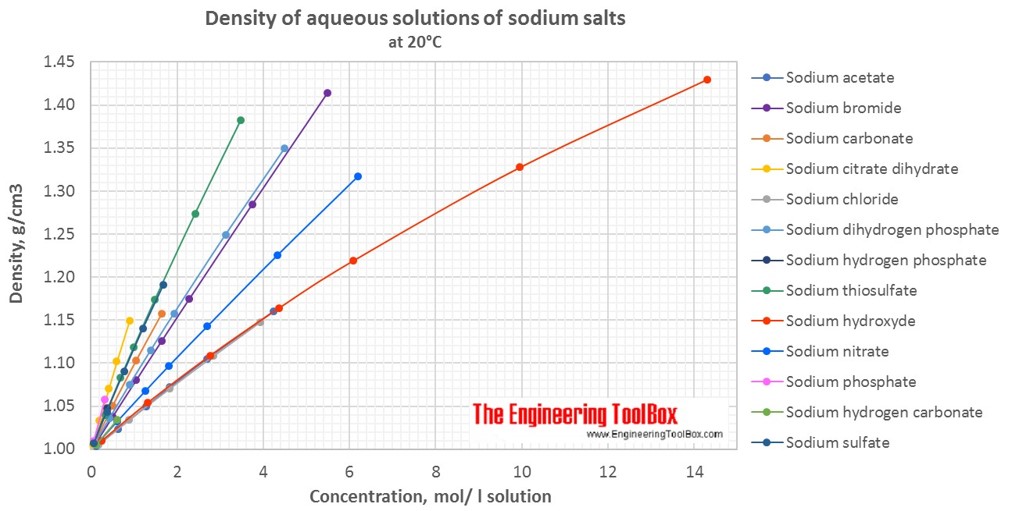 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
0971 20 C oxidation states 1 1 rare electron configuration. Do not put any ointments oils or medication in the victims eyes without specific instructions from a physician. It melts without decomposition at 852 o C. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. Water CAS 7732-18-5 Balance See Section 8 for exposure guidelines Section 3.
Source: chemspider.com
Volume of distilled water. 02M hydrochloric acid standardization against sodium carbonate. Consequently calcium sulphate must be reacted upon. When dissolving solid NaOH in water or a strong acid. While weighing do not spill the substance on.
Source:
The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. HClO 4 71 113 Molar Strength 70-72 Density 16 Molecular Weight 10046 1 liter 1600 gm 1136 gm HClO 4 71 113 moles 113 Molar. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. National Toxicology Program Chemical Repository Database. The general method of ester preparation can be.
 Source: www2.csudh.edu
Source: www2.csudh.edu
In 1998 in terms of. 120 at 20ºC 68ºF Odor. Health - 3 Flammability - 0 Reactivity - 2. See also density of aqueous solutions of inorganic chlorides inorganic potassium salts some other inorganic substances organic acids and organic. HAZARDS IDENTIFICATION Cont EMERGENCY OVERVIEW.
 Source: www2.csudh.edu
Source: www2.csudh.edu
9781 C 208 F boiling point. Gloves The Synthesis of Ethyl Ethanoate. Soda ash is the trade name for sodium carbonate a chemical refined from the mineral trona or sodium-carbonate-bearing brines both referred to as natural soda ash or manufactured from one of several chemical processes referred to as synthetic soda ash. Boiling Point 122C 252F Phosphoric Acid. When titrated with hydrochloric acid carbonate decomposes yielding carbon dioxide and water.
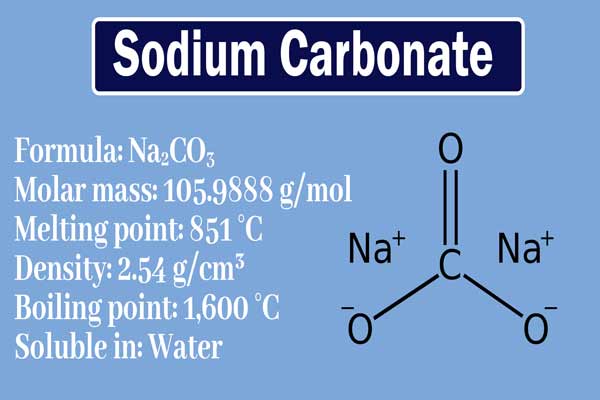 Source: chemistrypage.in
Source: chemistrypage.in
Sodium carbonate CAS 497-19-8 anhydrous for analysis EMSURE ISO - Find MSDS or SDS a COA data sheets and more information. 1388 C 2530 F. Its boiling point is 101. PH 401 500 700 and 1001 buffers are color coded for ease of use. HClO 4 71 113 Molar Strength 70-72 Density 16 Molecular Weight 10046 1 liter 1600 gm 1136 gm HClO 4 71 113 moles 113 Molar.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium carbonate solution boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.