Sodium carbonate boil point
Home » datasheet » Sodium carbonate boil pointSodium carbonate boil point
Sodium Carbonate Boil Point. Salt is a mineral composed primarily of sodium chloride NaCl a chemical compound belonging to the larger class of salts. Typically this is done by adding sodium hydroxide but calcium carbonate or calcium hydroxide can also be used depending on what pH youre aiming for. The test sample is titrated against carbonate-free 01 M sodium hydroxide solution using 4-5 drops of neutralized phenolphthalein indicator. Remove from heat and allow to cool.
 Sodium Carbonate Assignment Point From assignmentpoint.com
Sodium Carbonate Assignment Point From assignmentpoint.com
The result is. To further reduce the water the gypsum is reheated to about 402 degrees Fahrenheit 204 degrees Celsius at which point it is removed from the kettle. As an alternative a pH meter may be used and the sample titrated to pH 83. This means that calcium needs more than 14 times of needed heat to boil water. For example sodium chloride melts at 801 C and boils at 1413 C. The end point is 4210 mL of NaOH.
Salt is essential for life in general and.
What happens to melting point in s block. Turkey Wrapped In Bacon With Added Water 20Turkey Breast Pork Belly Water Salt Glucose Stabiliser Disodium. Add a few drops of bromophenol blue indicator to the flask. This recipe makes a single 8 oz serving. So melting point of Mg is higher than Na. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.
 Source: assignmentpoint.com
Source: assignmentpoint.com
Salt came from seawater while soda came from the Natron Valley in Egypt or from the ash of certain plants. The chemical properties of calcium include its melting point which is 8390 - 2C while its boiling point is 14840ºC with a valence of 2. The declared weight 2205kg includes the weight of the Champagne at approx. If you study s block elements in same period you will see alkaline earth metal group 2 has a higher melting point than alkali metal group 1 because. Salt in the form of a natural crystalline mineral is known as rock salt or haliteSalt is present in vast quantities in seawaterThe open ocean has about 35 grams 12 oz of solids per liter of sea water a salinity of 35.
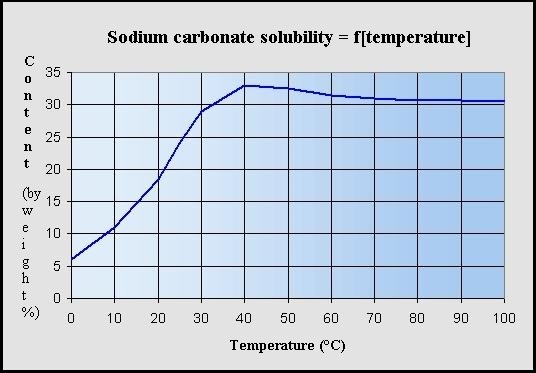 Source: hydro-land.com
Source: hydro-land.com
In equation 1 the acid is HCl called hydrochloric acid and the base is NaOH called sodium hydroxide. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances. So melting point of Mg is higher than Na. Place a watch glass on top of the beaker. 2012 a A metal which is preserved in kerosene b A lustrous coloured non metal c A metal which can melt while kept on palm d A metal which is a poor conductor of heat.

There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances. Remove from heat and allow to cool. Add 30 ml of deionized water and 20 g of anhydrous sodium carbonate. The main component of chalk is calcium carbonate. Sodium hydrogen carbonate reacts with carboxylic acids to give the sodium salt of the acid and liberates carbon dioxide.
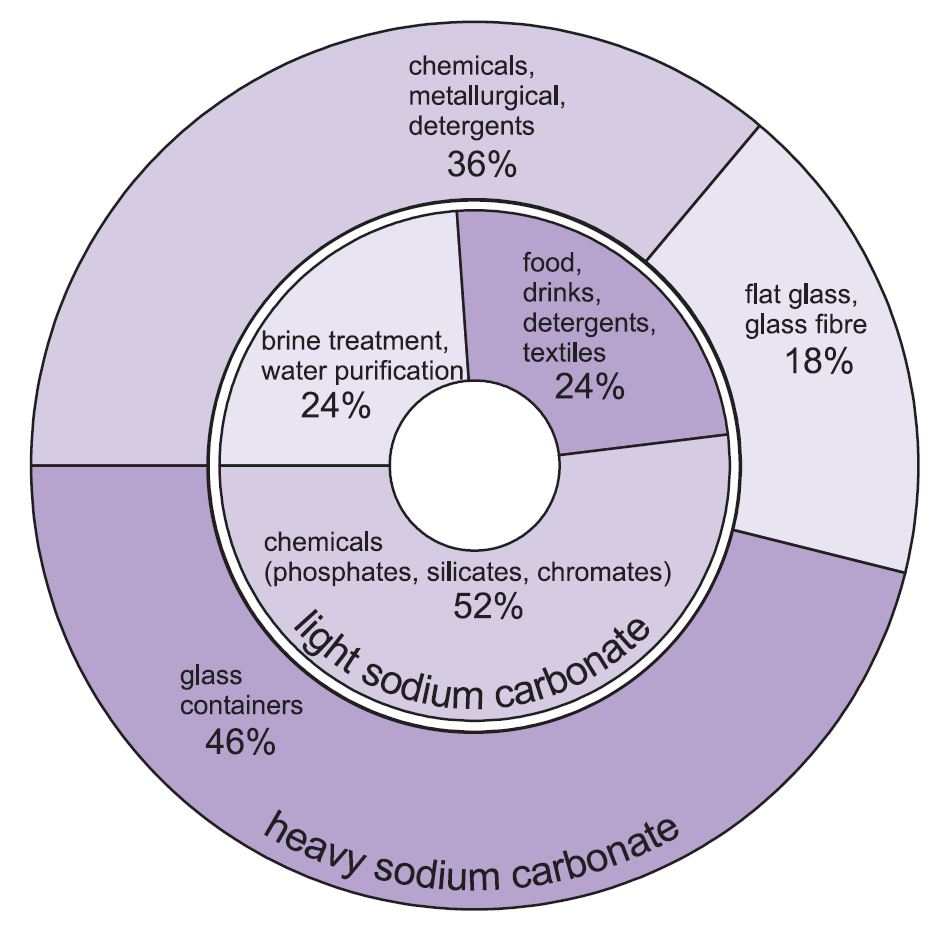
The basic principle of all low-pressure freshwater generators is that the boiling point of the water can be reduced by reducing the pressure of the surrounding atmosphere. Bring water to boil. As a comparison the molecular compound water melts at 0 C and boils at 100 C In solid form an ionic compound is not electrically conductive because its ions are unable to flow electricity is the flow of. The declared weight 2205kg includes the weight of the Champagne at approx. The test sample is titrated against carbonate-free 01 M sodium hydroxide solution using 4-5 drops of neutralized phenolphthalein indicator.
 Source: en.wikipedia.org
Source: en.wikipedia.org
So melting point of Mg is higher than Na. Building your exercise program up slowly into more cardiovascular exercise is a. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. The titration proceeds until the equivalence point is reached where the number of moles of acid is equal to the number of moles of base. Sodium hydrogen carbonate reacts with carboxylic acids to give the sodium salt of the acid and liberates carbon dioxide.
 Source: www2.csudh.edu
Source: www2.csudh.edu
So melting point of Mg is higher than Na. Place the beaker on a wire gauze supported by a ring on a ring stand. 2012 a A metal which is preserved in kerosene b A lustrous coloured non metal c A metal which can melt while kept on palm d A metal which is a poor conductor of heat. In equation 1 the acid is HCl called hydrochloric acid and the base is NaOH called sodium hydroxide. The Learning Center provides on-site activities that enable visiting students to gain first-hand exposure to Argonnes unique culture of innovation and.
Source: chemspider.com
As a comparison the molecular compound water melts at 0 C and boils at 100 C In solid form an ionic compound is not electrically conductive because its ions are unable to flow electricity is the flow of. Add 30 ml of deionized water and 20 g of anhydrous sodium carbonate. Beyond this point Festive Food to Order products cannot be changed but non Festive Food to Order products can still be amended. I Sodium ii Mercury. The titration proceeds until the equivalence point is reached where the number of moles of acid is equal to the number of moles of base.
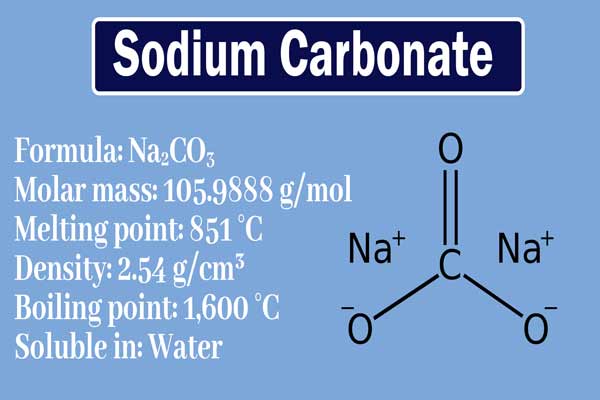 Source: chemistrypage.in
Source: chemistrypage.in
In equation 1 the acid is HCl called hydrochloric acid and the base is NaOH called sodium hydroxide. Salt in the form of a natural crystalline mineral is known as rock salt or haliteSalt is present in vast quantities in seawaterThe open ocean has about 35 grams 12 oz of solids per liter of sea water a salinity of 35. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Bring water to boil. They were scraped from the walls of latrines manufactured from clay and sulfuric acid and collected as wood ash respectively.
 Source: assignmentpoint.com
Source: assignmentpoint.com
Nickel carbonate NiCO 3 decomposes to NiO and CO 2 on heating. Water can be boiled at low temperatures by maintaining a low pressure say 50 degrees CelsiusThe heat source for the freshwater generator could be waste heat rejected by main engine jacket cooling water. 2012 a A metal which is preserved in kerosene b A lustrous coloured non metal c A metal which can melt while kept on palm d A metal which is a poor conductor of heat. Gently boil the contents of the beaker for 10 minutes using a micro burner. As a comparison the molecular compound water melts at 0 C and boils at 100 C In solid form an ionic compound is not electrically conductive because its ions are unable to flow electricity is the flow of.
 Source: researchgate.net
Source: researchgate.net
Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. When making baked goods this process is called chemical. The basic solution now theoretically been stripped of DMT. Salt is a mineral composed primarily of sodium chloride NaCl a chemical compound belonging to the larger class of salts. The titration proceeds until the equivalence point is reached where the number of moles of acid is equal to the number of moles of base.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium carbonate boil point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.