Sodium borohydride melting point
Home » datasheet » Sodium borohydride melting pointSodium borohydride melting point
Sodium Borohydride Melting Point. It also includes a recrystallization in an organic solvent. 실험실에서 아세트아미노펜을 만들기 위해서는 페놀에 묽은 황산과 함께 질산나트륨을 첨가하면 페놀의 벤젠 고리가 니트로화 반응을 하여 p-니트로페놀p-nitrophenol과 부산물로 o-니트로페놀o-nitrophenol이 생성된다. Showing the melting point of sodium as a function of potassium concentration. Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb.
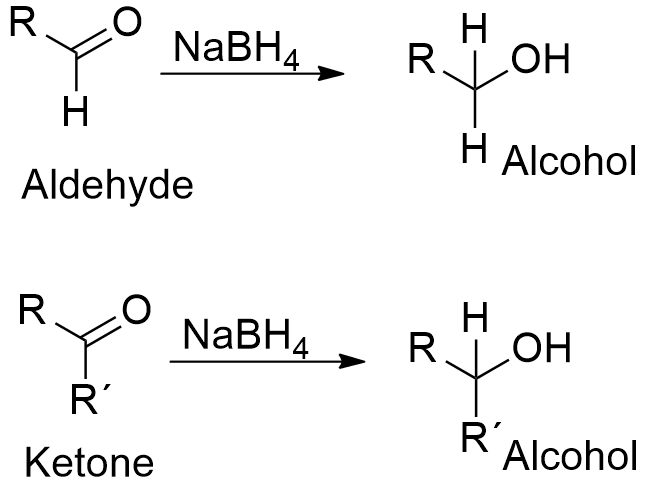
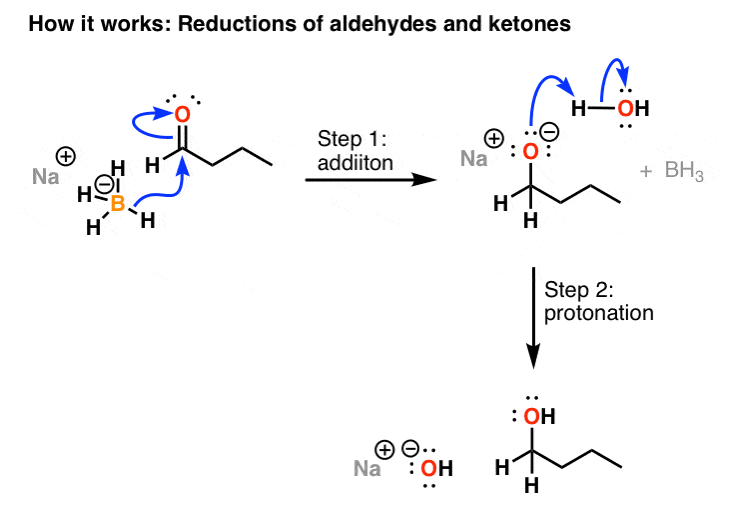
Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb. It wouldnt be an exaggeration to state that you cant master Organic Chemistry without a good understanding of Acid-Base Chemistry. In the borohydride reduction the hydroxylic solvent system achieves this hydrolysis automatically. Liquid sodium is used as a heat transfer fluid in some types of nuclear reactors because it has the high thermal. Reduction of a ketone using sodium borohydride. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized.
Structure of Sodium Chloride NaCl.
Boiling Point of sodium chloride. Tert-butyl group and an extended carbon chain might be more potent and specific SL biosynthesis inhibitors. The specific examples on page two will give you a pattern for determining the compound with a higher boiling or melting point. Reduction of a ketone using sodium borohydride. It is used in the soda ash industry to produce sodium carbonate. It also includes a recrystallization in an organic solvent.
 Source: softschools.com
Source: softschools.com
Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. Partial reduction of carboxylic acids directly to aldehydes is not possible but such conversions have been achieved in two steps by way. It also includes a recrystallization in an organic solvent. Sodium does not react with. Having just talked about the oxidation ladder it makes sense to start going into.

Select Chapter 2 - Chemical Methods Used in Purification. Reaction of sodium or potassium hydroxide on aluminum electrolysis of water or displacement from acids by certain metals. P-니트로페놀을 정제하여 수소화붕소나트륨sodium borohydride을 첨가하면 4-아미노. Liquid sodium is used as a heat transfer fluid in some types of nuclear reactors because it has the high thermal. It is used in fire extinguishers.
 Source: studylib.net
Source: studylib.net
The purified product should have spectroscopic properties which indicate that the traces of impurities left in the sample are of acceptable levels for the intended purpose. This summary guide will give all you need to know in this essential chapter. The specific examples on page two will give you a pattern for determining the compound with a higher boiling or melting point. Sodium borohydride NaBH 4 does not reduce carboxylic acids. Tert-butyl group and an extended carbon chain might be more potent and specific SL biosynthesis inhibitors.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The last reaction shows how an acetal derivative may be used to prevent reduction of a carbonyl function in this case a ketone. The presence of low melting point Na 2 Se x phases as grain growth promoters has been previously reported in chalcogenide solar cell absorbers. F2 atom closest to negative side. Sodium borohydride NaBH 4 does not reduce carboxylic acids. Reduction of a ketone using sodium borohydride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Based on this Effects of TIS13 derivatives on 29-epi-5-deoxystrigol notion we synthesized TIS108 Fig. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. The last reaction shows how an acetal derivative may be used to prevent reduction of a carbonyl function in this case a ketone. The specific examples on page two will give you a pattern for determining the compound with a higher boiling or melting point. Metallic sodium is used mainly for the production of sodium borohydride sodium azide indigo.
 Source: chemyq.com
Source: chemyq.com
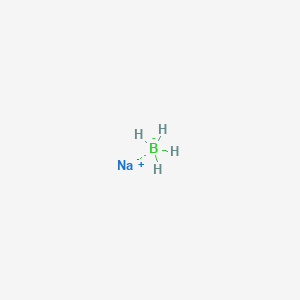
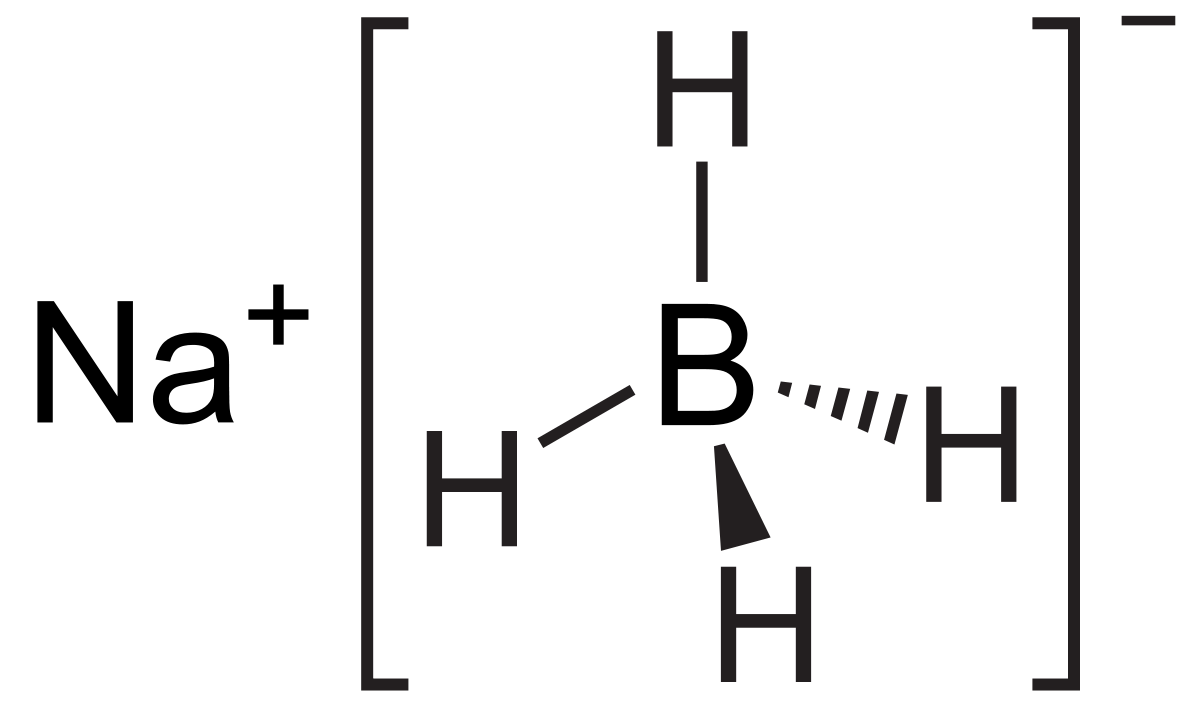
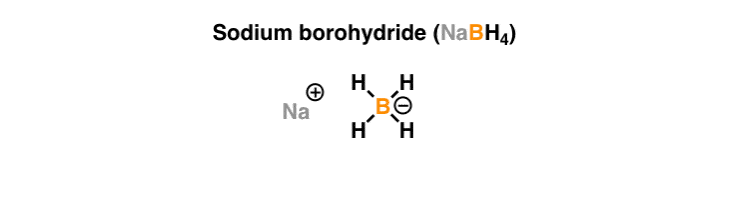
Partial reduction of carboxylic acids directly to aldehydes is not possible but such conversions have been achieved in two steps by way. 8829 C 1621 F specific gravity. Sodium borohydride also known as sodium tetrahydridoborate and sodium tetrahydroborate is an inorganic compound with the formula Na BH 4This white solid usually encountered as a powder is a reducing agent that finds application in chemistry both in the laboratory and on an industrial scale. ALL YOUR PAPER NEEDS COVERED 247. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Uses of Sodium Chloride NaCl It is used in medicine Saline solution in nasal spray. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized. Having just talked about the oxidation ladder it makes sense to start going into. However hydrogen gas is liberated and salts of the acid are formed. The lithium sodium boron and aluminum end up as soluble inorganic salts.
 Source: en.wikipedia.org
Source: en.wikipedia.org
TIS108 was 100-fold epi-5DS levels and growth retardation. Uses of Sodium Chloride NaCl It is used in medicine Saline solution in nasal spray. Sodium Borohydride NaBH 4 For Reduction Of Aldehydes And Ketones And Demercuration. 1 g of the above betaine in a mixture of 20 ml of ethanol and 5 ml of water is treated with 008 g of sodium borohydride and this solution is refluxed for 10 min and kept at 25 for 1 hour after the reflux is finished. It wouldnt be an exaggeration to state that you cant master Organic Chemistry without a good understanding of Acid-Base Chemistry.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Structure of Sodium Chloride NaCl. Liquid hydrogen is important in cryogenics and in the study of superconductivity as its melting point is only 20 degrees above absolute zero. Sodium is reactive soft metal with a low melting point and most important of all the alkaline metals from the commercial point of view. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. Sodium borohydride also known as sodium tetrahydridoborate and sodium tetrahydroborate is an inorganic compound with the formula Na BH 4This white solid usually encountered as a powder is a reducing agent that finds application in chemistry both in the laboratory and on an industrial scale.
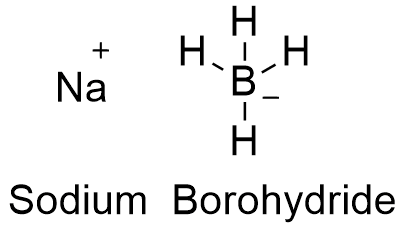 Source: softschools.com
Source: softschools.com
Crystallization may be repeated until the substance has a constant melting point and until it distils repeatedly within a narrow specified temperature range. No matter what kind of academic paper you need it is simple and affordable to place your order with Achiever Essays. Hydrogen is the primary. Showing the melting point of sodium as a function of potassium concentration. Tritium is readily produced in nuclear reactors and is used in the production of the hydrogen bomb.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium borohydride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.