Sodium bisulfite boiling point
Home » datasheet » Sodium bisulfite boiling pointSodium bisulfite boiling point
Sodium Bisulfite Boiling Point. Mix 1-12 tablespoons of Sodium Bisulfate in 1-gallon of water. Sodium Bisulfite Solution Manufacturer. Adding preservatives is the chemical methods of food preservation. A 25 aqueous sodium hydroxide solution 170 g was added to the reaction mixture at 60 to 63 C and the mixture was stirred at the same temperature for 4 hours.
 4435 33 0 Sodium Bisulfite Addn Compd C4h10o4s Formula Nmr Boiling Point Density Flash Point From guidechem.com
4435 33 0 Sodium Bisulfite Addn Compd C4h10o4s Formula Nmr Boiling Point Density Flash Point From guidechem.com
Covalent compounds sometimes dissolve well in water eg hydrogen chloride HCl and sometimes. NFPA 704 fire diamond 2. Available directly through Excalibur. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Nair B Elmore AR. Boiling point 78158C Flammability Readily flammable burning with a blue smokeless flame.
Nair B Elmore AR.
100 454 Sodium. The reactors operating temperature is much less than the boiling point of sodium. Boiling point 78158C Flammability Readily flammable burning with a blue smokeless flame. Acetone is valuable solvent component in acrylicnitrocellulose automotive lacquers. 1000 454 Sodium hypochlorite. 5000 2270 Sodium chromate.
 Source: foodadditives.net
Source: foodadditives.net
The acids HX produced have a lower boiling point than the reactants and are separated from the reaction mixture by distillation. A 25 aqueous sodium hydroxide solution 170 g was added to the reaction mixture at 60 to 63 C and the mixture was stirred at the same temperature for 4 hours. Hence it is used in cooling a fast reactor. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O. Acetone is valuable solvent component in acrylicnitrocellulose automotive lacquers.
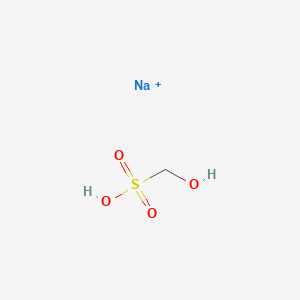
Sodium bisulfite is designated as a hazardous substance under section 311b2A of the Federal Water Pollution Control Act and further regulated by the Clean Water Act Amendments of 1977 and 1978. Specific gravity 0811908139 at 208CNote the above typical properties are for alcohol ethanol 95 or96 vv. 100 454 Sodium. Slightly soluble in alcohol. The reactors operating temperature is much less than the boiling point of sodium.
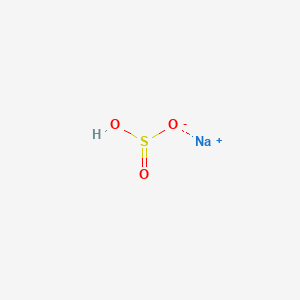
These regulations apply to discharges of this substance. Covalent compounds tend to be soft and have relatively low melting and boiling points. 1000 454 Sodium hypochlorite. Nair B Elmore AR. Covalent bonds have low melting and boiling point.

This designation includes any isomers and hydrates as well as any solutions and mixtures containing this substance. Hydrated sodium bisulfate dehydrates at 58 C 136 F at which point it separates from the water molecule attached to. Sodium bisulfite is designated as a hazardous substance under section 311b2A of the Federal Water Pollution Control Act and further regulated by the Clean Water Act Amendments of 1977 and 1978. Specific gravity 0811908139 at 208CNote the above typical properties are for alcohol ethanol 95 or96 vv. Various Please Contact Supplier for Further Information Supplier.

Slightly soluble in alcohol. Water a liquid composed of covalently bonded molecules can also be used as a test substance for other ionic and covalently compounds. Water was added to the reaction mixture and the aqueous layer was removed. Hydrated sodium bisulfate dehydrates at 58 C 136 F at which point it separates from the water molecule attached to. HClaq hydrochloric acid HFaq hydrofluoric acid H2Saq hydrosulfuric acid Comment.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Acetone is valuable solvent component in acrylicnitrocellulose automotive lacquers. Various industries including petroleum chemicals soaps textiles and paper have been using sodium compounds on a large-scale to carry out their important. Although sodium uses are limited its compounds have a wide range of uses. Hydrated sodium bisulfate dehydrates at 58 C 136 F at which point it separates from the water molecule attached to. HClaq hydrochloric acid HFaq hydrofluoric acid H2Saq hydrosulfuric acid Comment.
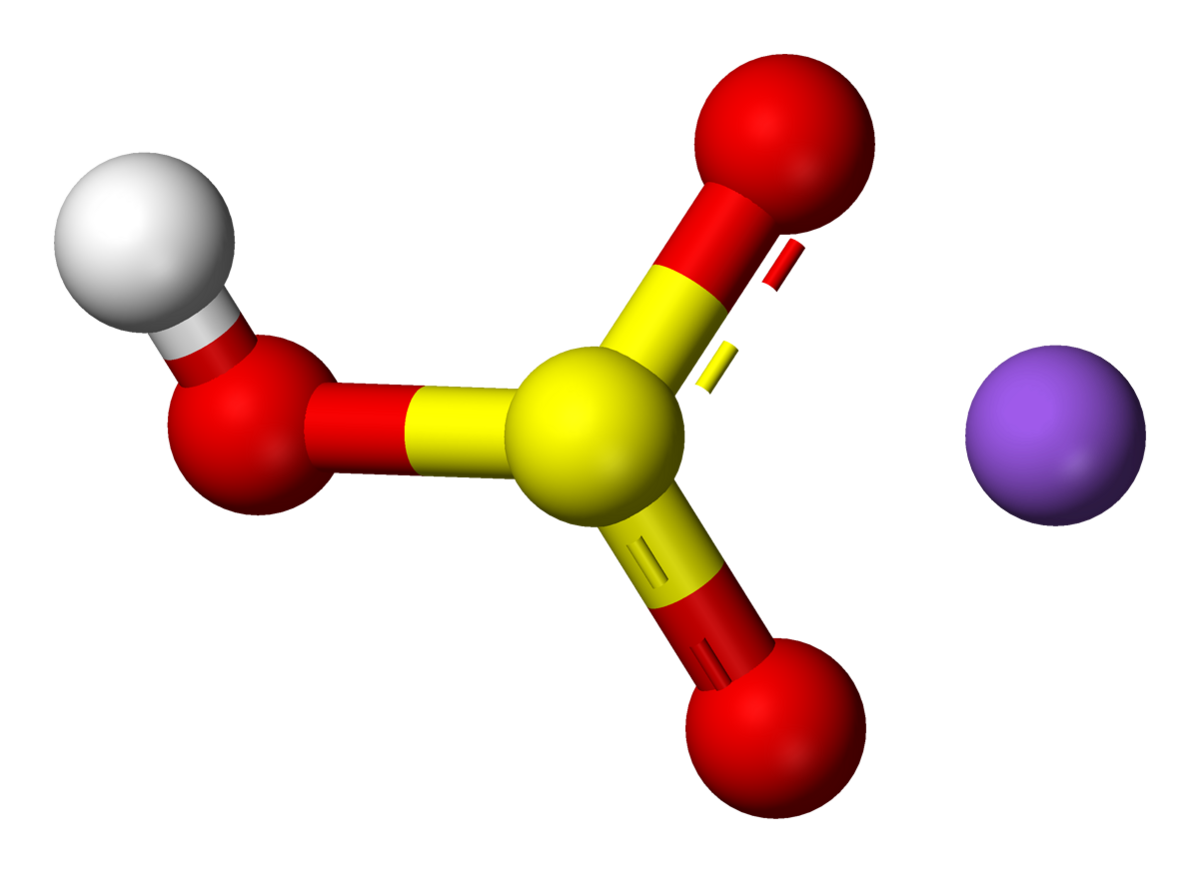 Source: en.wikipedia.org
Source: en.wikipedia.org
941 Redox Titration Curves. This designation includes any isomers and hydrates as well as any solutions and mixtures containing this substance. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O. Acetone is valuable solvent component in acrylicnitrocellulose automotive lacquers. GHS hazard statements.
 Source: sciencemadness.org
Source: sciencemadness.org
5000 2270 Sodium chromate. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The reactors operating temperature is much less than the boiling point of sodium. Available directly through Excalibur. For example in the ionic compound sodium chloride NaCl the chlorine ion Cl 1 gains one electron that was given by HCl hydrogen chloride HF hydrogen fluoride H2S hydrogen sulfide Rule 2 When dissolved in H2O.
 Source: guidechem.com
Source: guidechem.com
Flash point 148C closed cup. Various industries including petroleum chemicals soaps textiles and paper have been using sodium compounds on a large-scale to carry out their important. Hence it is used in cooling a fast reactor. Water a liquid composed of covalently bonded molecules can also be used as a test substance for other ionic and covalently compounds. Int J Toxicol 22 Suppl 2 63-88 2003 Cosmetic Ingredients Review Expert Panel report.

The main target users are workers and those responsible for occupational safety and health. Although sodium uses are limited its compounds have a wide range of uses. It is the most effective and least expensive anti-oxidant. Slightly soluble in alcohol. BHA and BHT are antioxidants.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium bisulfite boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.