Sodium bicarbonate used in boiling point
Home » datasheet » Sodium bicarbonate used in boiling pointSodium bicarbonate used in boiling point
Sodium Bicarbonate Used In Boiling Point. It is also employed in. This compound is insoluble in ethanol and slightly soluble in methanol and acetone. WATER POLLUTION 61 Aquatic Toxicity. Upper Explosive Limit UEL.
 Sodium Bicarbonate Wikipedia From en.wikipedia.org
Sodium Bicarbonate Wikipedia From en.wikipedia.org
Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions. Chemical Properties of Sodium Oxide Na 2 O. Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate. 3 Basic salt normally aqueous bicarbonate of potassium potash sodium bicarbonate sodium carbonate sodium phosphate etc. Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF. Currently not available 63 Biological Oxygen Demand BOD.
NaHCO3 has a white crystalline appearance.
Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml. Upper Explosive Limit UEL. Physical Properties of Sodium Oxide Na 2 O. In the manufacture. WATER POLLUTION 61 Aquatic Toxicity. Not pertinent 45 Special Hazards of Combustion Products.
 Source: in.pinterest.com
Source: in.pinterest.com
The softening point is also related to the intrinsic viscosity hardness and brittleness of resins. Physical Properties of Sodium Oxide Na 2 O. Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml. Sodium silicate is used to react selectively with magnesium hardness. Though potassium is the better ion in most cases sodium is chosen for its lower price and atomic weight.
 Source: worldofchemicals.com
Source: worldofchemicals.com
Sodium oxide reacts with carbon dioxide to form. The resins are non-crystalline amorphous materials which soften gradually over a range of temperatures. Baking soda actually contains sodium its in the name and its chemical name is sodium bicarbonate where Im sure youve come across it in baking or cooking where it undergoes thermal decomposition at above 70C to release carbon dioxide - which then makes. Sodium hydroxide is used in many industries. 3 Basic salt normally aqueous bicarbonate of potassium potash sodium bicarbonate sodium carbonate sodium phosphate etc.
 Source: en.wikipedia.org
Source: en.wikipedia.org
WATER POLLUTION 61 Aquatic Toxicity. The resins are non-crystalline amorphous materials which soften gradually over a range of temperatures. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc. Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other edematous or sodium-retaining conditions. Upper Explosive Limit UEL.
 Source: sciencetrends.com
Source: sciencetrends.com
A few other important properties of sodium hydrogen carbonate are listed below. S Density g cm 3 097 Atomic number. Sodium chloride is extensively used for anti-icing and de-icing and as a preservative. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc. Without decomposition and with a boiling point of 1388 C 2530 F.
Source: chemspider.com
Its slight alkalinity makes it useful in treating gastric or urinary hyperacidity and acidosis. 7295 JmolK Hydrogen Bond Acceptor. Sodium oxide reacts with carbon dioxide to form. NaOH is insoluble in ether and other non-polar solvents. Along with potassium many important medicines have sodium added to improve their bioavailability.
 Source: slideplayer.com
Source: slideplayer.com
Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF. The resins are non-crystalline amorphous materials which soften gradually over a range of temperatures. Without decomposition and with a boiling point of 1388 C 2530 F. JOSÉ MIGUEL MARTÍN-MARTÍNEZ in Adhesion Science and Engineering 2002. Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF.
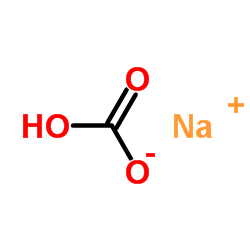 Source: chemsrc.com
Source: chemsrc.com
Similar to the hydration of sulfuric acid dissolution of solid sodium hydroxide in water is a. Though potassium is the better ion in most cases sodium is chosen for its lower price and atomic weight. A few other important properties of sodium hydrogen carbonate are listed below. And in patients receiving corticosteroids or corticotropin since each gram of sodium bicarbonate contains about 12 mEq of sodium. 882940C 1621292F 1156090 K Block.
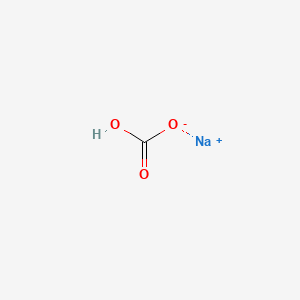
NaHCO3 has a white crystalline appearance. Symptoms of systemic benzoate toxicity resemble those. Along with potassium many important medicines have sodium added to improve their bioavailability. Not pertinent 45 Special Hazards of Combustion Products. In patients with renal insufficiency especially those with severe insufficiency such as oliguria or anuria.
 Source: thoughtco.com
Source: thoughtco.com
Baking soda actually contains sodium its in the name and its chemical name is sodium bicarbonate where Im sure youve come across it in baking or cooking where it undergoes thermal decomposition at above 70C to release carbon dioxide - which then makes. May be prepared also from 154 g chlorinated lime 30 available chlorine 77 g anhydrous sodium carbonate and 64 g sodium. The Physical Property fields include properties such as vapor pressure and boiling point as well as explosive limits and toxic exposure thresholds The information in CAMEO Chemicals comes from a variety of data sources. In the manufacture. Therefore the softening point is controlled by the average molecular weight of the resin.
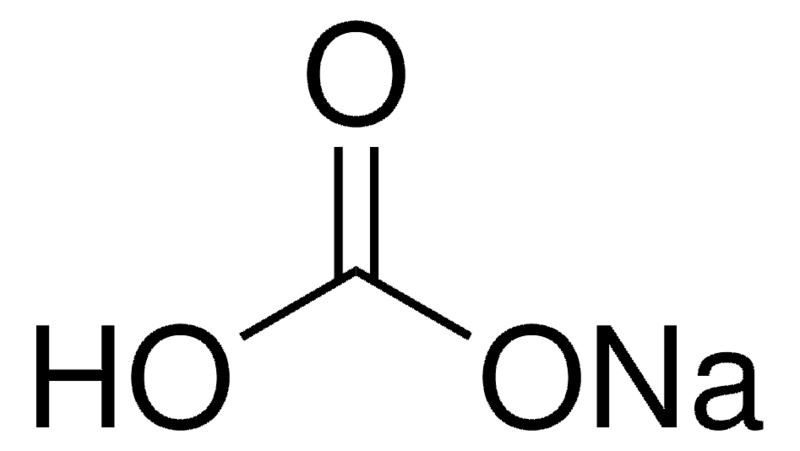 Source: sigmaaldrich.com
Source: sigmaaldrich.com
3 Basic salt normally aqueous bicarbonate of potassium potash sodium bicarbonate sodium carbonate sodium phosphate etc. NaOH is insoluble in ether and other non-polar solvents. The softening point is also related to the intrinsic viscosity hardness and brittleness of resins. Physical Properties of Sodium Oxide Na 2 O. Chemical Properties of Sodium Oxide Na 2 O.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium bicarbonate used in boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.