Sodium bicarbonate boiling melting point
Home » datasheet » Sodium bicarbonate boiling melting pointSodium bicarbonate boiling melting point
Sodium Bicarbonate Boiling Melting Point. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Rapid correction of acidosis with sodium bicarbonate in patients with diabetic ketoacidosis may cause hypokalemia paradoxical acidosis in cerebrospinal fluid CSF since carbon dioxide diffuses more rapidly into CSF than does bicarbonate and lactic acidosis since increased pH increases hemoglobin-oxygen affinity which when combined with erythrocyte 23-diphosphoglycerate deficiency in these. This method also allows a mesophase pitch to be studied in the liquid crystalline phase Fig. The readiest means of preparing hydrochloric acid gas consists of the application of heat to its strong aqueous solution in.
Sodium Bicarbonate Chnao3 Chemspider From chemspider.com
Vapor Density Relative to Air. Preparation of Hydrochloric acid HCl. Rapid correction of acidosis with sodium bicarbonate in patients with diabetic ketoacidosis may cause hypokalemia paradoxical acidosis in cerebrospinal fluid CSF since carbon dioxide diffuses more rapidly into CSF than does bicarbonate and lactic acidosis since increased pH increases hemoglobin-oxygen affinity which when combined with erythrocyte 23-diphosphoglycerate deficiency in these. Aluminium hydroxide is prepared at the treatment plant from aluminium sulfate by reacting it with sodium hydroxide or bicarbonate. Al 2 SO 4 3 6 NaOH 2 AlOH 3 3 Na 2 SO 4 Al 2 SO 4 3 6 NaHCO 3 2 AlOH 3 3 Na 2 SO 4 6 CO 2 Saponification. 2159 NTP 1992 Boiling Point.
Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each.
A spectrum of an isotropic pitch is shown in Fig. Atomic-resolution real-time video imaging. Al 2 SO 4 3 6 NaOH 2 AlOH 3 3 Na 2 SO 4 Al 2 SO 4 3 6 NaHCO 3 2 AlOH 3 3 Na 2 SO 4 6 CO 2 Saponification. Solid sodium chloride has a melting point of 801 C. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Sodium hydroxide can be used for the base-driven hydrolysis of.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A spectrum of an isotropic pitch is shown in Fig. 8401 NTP 1992 Water Solubility. In order to understand the possible role of reactive intermediates in the formation of tissue-specific DNA damage by chromiumVI electron paramagnetic resonance spectroscopy was used to study the in vivo formation of chromiumV in the liver and red blood cells of 14 day chick embryos following treatment with chromiumVIIn vivo administration of sodium dichromate onto the inner shell. Vapor Density Relative to Air. Al 2 SO 4 3 6 NaOH 2 AlOH 3 3 Na 2 SO 4 Al 2 SO 4 3 6 NaHCO 3 2 AlOH 3 3 Na 2 SO 4 6 CO 2 Saponification.
Source: chemspider.com
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Decomposes at 228 F NTP 1992 Vapor Pressure. 596 K Boiling point. The Physical Property fields include properties such as vapor pressure and boiling point as well. For pitch-like materials the best temperature for measurement is the softening point plus 100C.
 Source: sciencetrends.com
Source: sciencetrends.com
8007 C 14733 F. It is used in the processing of leather production of gelatin. Preparation of Hydrochloric acid HCl. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. Vapor Density Relative to Air.
 Source: slideplayer.com
Source: slideplayer.com
44527 F and decreases to 0069 at 314 K 41 C. 323 C 613 F. Aluminium hydroxide is prepared at the treatment plant from aluminium sulfate by reacting it with sodium hydroxide or bicarbonate. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium hydroxide can be used for the base-driven hydrolysis of. 10738 K Boiling point. For pitch-like materials the best temperature for measurement is the softening point plus 100C. 323 C 613 F. It also decreases with doping.
 Source: chemsrc.com
Source: chemsrc.com
8007 C 14733 F. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. 8007 C 14733 F. 323 C 613 F. In order to understand the possible role of reactive intermediates in the formation of tissue-specific DNA damage by chromiumVI electron paramagnetic resonance spectroscopy was used to study the in vivo formation of chromiumV in the liver and red blood cells of 14 day chick embryos following treatment with chromiumVIIn vivo administration of sodium dichromate onto the inner shell.
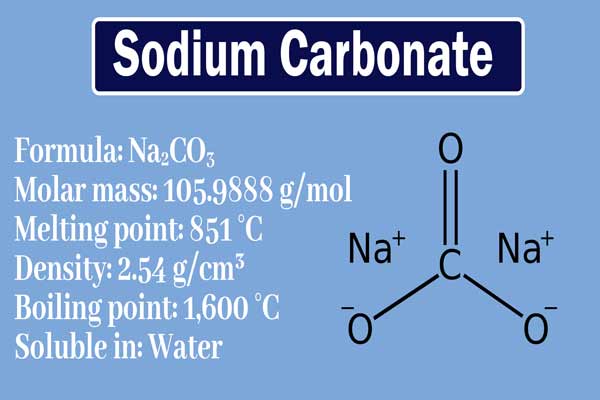 Source: chemistrypage.in
Source: chemistrypage.in
Decomposes at 228 F NTP 1992 Vapor Pressure. 1 in 10 at 77 F NTP 1992. Hydrochloric acid Structure HCl. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Rapid correction of acidosis with sodium bicarbonate in patients with diabetic ketoacidosis may cause hypokalemia paradoxical acidosis in cerebrospinal fluid CSF since carbon dioxide diffuses more rapidly into CSF than does bicarbonate and lactic acidosis since increased pH increases hemoglobin-oxygen affinity which when combined with erythrocyte 23-diphosphoglycerate deficiency in these.
 Source: in.pinterest.com
Source: in.pinterest.com
Atomic-resolution real-time video imaging. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Aluminium hydroxide is prepared at the treatment plant from aluminium sulfate by reacting it with sodium hydroxide or bicarbonate. The readiest means of preparing hydrochloric acid gas consists of the application of heat to its strong aqueous solution in. Atomic-resolution real-time video imaging.
Preparation of Hydrochloric acid HCl. For pitch-like materials the best temperature for measurement is the softening point plus 100C. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. The readiest means of preparing hydrochloric acid gas consists of the application of heat to its strong aqueous solution in. The Physical Property fields include properties such as vapor pressure and boiling point as well.
 Source: slideplayer.com
Source: slideplayer.com
It is used in the processing of leather production of gelatin. 8007 C 14733 F. When a sample has a softening or melting point it is possible to get a high-resolution spectrum by measuring the sample in a fused or molten state. Rapid correction of acidosis with sodium bicarbonate in patients with diabetic ketoacidosis may cause hypokalemia paradoxical acidosis in cerebrospinal fluid CSF since carbon dioxide diffuses more rapidly into CSF than does bicarbonate and lactic acidosis since increased pH increases hemoglobin-oxygen affinity which when combined with erythrocyte 23-diphosphoglycerate deficiency in these. Preparation of Hydrochloric acid HCl.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium bicarbonate boiling melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.