Sodium aluminum sulfate boiling point
Home » datasheet » Sodium aluminum sulfate boiling pointSodium aluminum sulfate boiling point
Sodium Aluminum Sulfate Boiling Point. 2 AlOH 3 3 H 2 SO 4 Al 2 SO 4 3 6 H 2 O. 0095 1 nm. Laboratory chemicals Manufacture of substances 13 Details of the supplier of the safety data sheet Company. Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO.
Aluminium Sulfate Octadecahydrate 7784 31 8 From chemicalbook.com
The ICSC project is a common undertaking between the World Health Organization WHO and. Characteristics and Properties In. Boiling Point C Feature. Sodium SulfateAnhydrous Created by Global Safety Management Inc. 17 If the patient is comatose or seizing gastric lavage with sodium bicarbonate should be performed. To learn more about the Structure Properties Preparations Uses and FAQs of Sodium sulphate Visit BYJUS for detailed information.
Boiling Point C Feature.
Freely soluble in water insoluble in ethanol. CID 14178901 Aluminate AlO2- CID 5360545 Sodium Dates. And upon further weathering as bauxite and iron-rich laterite. Its a soft metal reactive and with a low melting point with a. Melting Point C Physical Form. At a temperature of 280 to 480 ºС the most stable reaction product is AlO OH.
Source: chemspider.com
Pure sodium hydroxide is a colorless crystalline solid that melts at 318 C 604 F without decomposition and with a boiling point of 1388 C 2530 F. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. The solution that results is then evaporated and allowed to crystallize. Not Applicable Odor Threshold. Standard potential - 051 V.
Source: chemicalbook.com
Electronic shell Ne 3s 1. To learn more about the Structure Properties Preparations Uses and FAQs of Sodium sulphate Visit BYJUS for detailed information. 17 If the patient is comatose or seizing gastric lavage with sodium bicarbonate should be performed. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. There are 3 valence electrons in the outer shell.
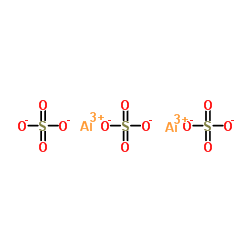 Source: chemsrc.com
Source: chemsrc.com
UOP624-14 Carbonyl Number by Chemical Analysis. Corrosive to metals and tissue. 0095 1 nm. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. 2467 C 4473 F specific gravity.
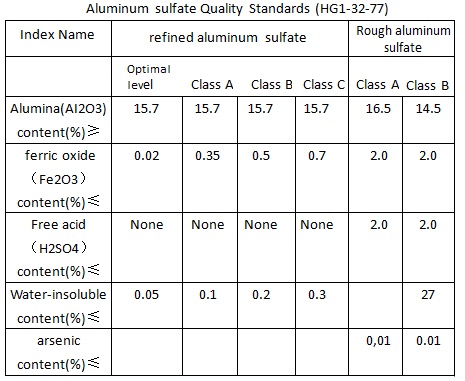 Source: chemicalbook.com
Source: chemicalbook.com
Aluminum occurs in igneous rocks chiefly as aluminosilicates in feldspars feldspathoids and micas. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Laboratory chemicals Manufacture of substances 13 Details of the supplier of the safety data sheet Company. SDBS has a relative density of 106. Its a soft metal reactive and with a low melting point with a.
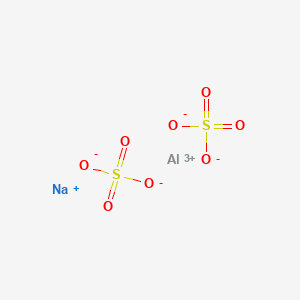
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. UOP649-10 Total Oxygen in Solid Semi-solid and High Boiling Point Liquid Hydrocarbons by Pyrolysis. Sodium SulfateAnhydrous ManufacturerSupplier Trade name. Aqueous solution SECTION 10. Melting point - the temperature at which a solid turns into a liquid.
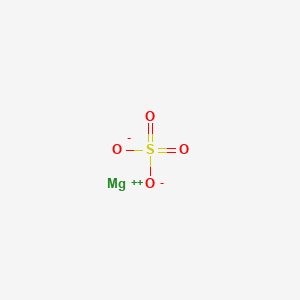
Aluminum is the second element in the thirteenth column of the periodic table. Boiling Point C Feature. At a temperature from room temperature to 280 ºС the most stable reaction product is Al OH 3. Sodium Sulfate Na2SO4 -Sodium sulfate is the sodium salt of sulfuric acid. 05 ppm SO2 Solubility in Water.
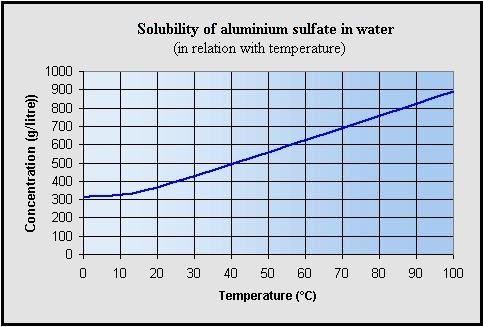 Source: hydro-land.com
Source: hydro-land.com
Preparation of Aluminium Sulphate. 1 g 75 ml at 25ºC. Hans Orsted in 1825 first isolated by Friedrich Wohler in 1827. Remember boiling water does not remove lead from water. Sulphur is a multivalent non-metal abundant tasteless.
 Source: en.wikipedia.org
Source: en.wikipedia.org
200 C MSDS DrugBank. Bauxite a mixture of. Sigma-Aldrich 3050 Spruce Street SAINT LOUIS MO. There are 3 valence electrons in the outer shell. Use your filter properly.
Laboratory chemicals Manufacture of substances 13 Details of the supplier of the safety data sheet Company. The main target users are workers and those responsible for occupational safety and health. It is highly soluble in water with a lower solubility in polar solvents such as ethanol and methanol. There are 3 valence electrons in the outer shell. Standard potential - 271 V.
 Source: chemsrc.com
Source: chemsrc.com
Energy of tird ionisation. Hans Orsted in 1825 first isolated by Friedrich Wohler in 1827. BECOMES ANHYD ABOUT 200 C. UOP649-10 Total Oxygen in Solid Semi-solid and High Boiling Point Liquid Hydrocarbons by Pyrolysis. Laboratory chemicals Manufacture of substances 13 Details of the supplier of the safety data sheet Company.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium aluminum sulfate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.