Sodium acetate melting point
Home » datasheet » Sodium acetate melting pointSodium acetate melting point
Sodium Acetate Melting Point. 58 Monday March 26 2012 Rules and Regulations Date of issue. The general method of ester preparation can be. To learn more about Sodium Acetate Preparation Properties Uses and FAQs Visit BYJUS for a. In the final part the students perform a melting point of the product and compare the.

Stopper the funnel and carefully mix the contents. For example the melting point of ice frozen water is 0 C. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. Sodium chemical element of the alkali metal group in the periodic table. It also includes a recrystallization in an organic solvent that is suitable to increase the skills of students that had limited contact with recrystallization techniques. Put the flask on a hot plate heat it gently and stir until the crystals of sodium acetate dissolve.
Identification Product form.
For example the melting point of ice frozen water is 0 C. Ann Arbor MI 48108 1. 0 360oC thermometers. Melting Point and Freezing Point. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. Chemical Product and Company Identification.

The melting point is specific for a given substance. Sodium Sulfate Anhydrous Safety Data Sheet according to Federal Register Vol. Remove the flask from the heat and let it cool slowly without disturbing it. It is widely used across a number of industrial sectors. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid.

The melting point is the highest temperature at which crystallization may occur. Sodium acetate anhydrous is used as an electrolyte replenisher in isosmotic solution for parenteral. When this solid melts the sodium acetate dissolves in the water that was trapped in the crystal to form a solution. Sodium Sulfate Anhydrous Safety Data Sheet according to Federal Register Vol. Melts ice and snow by lowering the freezing point of water.
 Source: sciencedirect.com
Source: sciencedirect.com
Creates heat as it turns to liquid. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. Chemical Product and Company Identification. 8829 C 1621 F specific gravity. Ann Arbor MI 48108 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Helps keep surfaces dry. The melting point depends on the pressure. Sodium Sulfate Anhydrous Safety Data Sheet according to Federal Register Vol. We say that such a body melts. Sodium Acetate Manufacturer Information Company Name.
 Source: researchgate.net
Source: researchgate.net
Corrosive and may damage concrete wood and plants. For example the melting point of ice frozen water is 0 C. Melts snow faster than. If a small crystal of sodium acetate trihydrate is added to the liquid however the. Because sodium is extremely reactive it never occurs in the free state in.
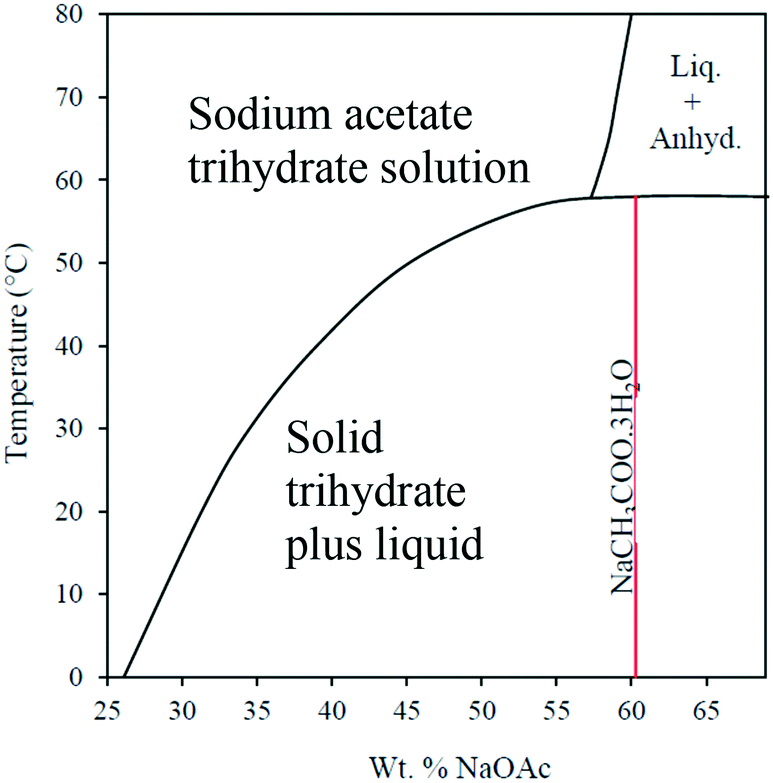 Source: pubs.rsc.org
Source: pubs.rsc.org
Allow the layers to separate completely remove the stopper from the funnel and then draw off the lower layer into a labeled 50 mL Erlenmeyer flask. It is hygroscopic in nature and easily soluble in water. But it often doesnt. The melting point is the highest temperature at which crystallization may occur. Substance Substance name.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting point is the highest temperature at which crystallization may occur. Because sodium is extremely reactive it never occurs in the free state in. Sodium chemical element of the alkali metal group in the periodic table. Sodium Acetate Anhydrous is the anhydrous sodium salt form of acetic acidSodium acetate anhydrous disassociates in water to form sodium ions Na and acetate ions. Corrosive and may damage concrete wood and plants.
 Source: sodiumacetate.weebly.com
Source: sodiumacetate.weebly.com
Chemical Product and Company Identification. We say that such a body melts. Product and Company Identification Product Code. It is also a temperature at which a solid crystal turns into a liquid. In the final part the students perform a melting point of the product and compare the.
 Source: sciencemadness.org
Source: sciencemadness.org
This may take up to two hours. 0971 20 C oxidation states 1 1 rare electron configuration. Product and Company Identification Product Code. For example the melting point of ice frozen water is 0 C. Remove the flask from the heat and let it cool slowly without disturbing it.
8829 C 1621 F specific gravity. It is also a temperature at which a solid crystal turns into a liquid. Ann Arbor MI 48108 1. Vent the funnel and then shake the mixture thoroughly venting often. Sodium Chloride NaCl Also known as ice melt saltrock salt.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium acetate melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.