Sodium acetate boiling point
Home » datasheet » Sodium acetate boiling pointSodium acetate boiling point
Sodium Acetate Boiling Point. It is hygroscopic in nature and easily soluble in water. Chemical Properties of Sodium Sulfite. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid to form a separate vapor phase above the liquid surface. Saturated sodium carbonate solution 1 Bottle.
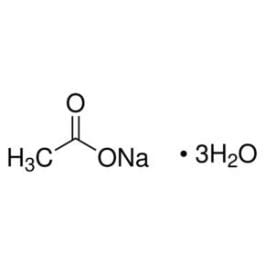 Sodium Acetate Trihydrate 32318 Honeywell Research Chemicals From lab.honeywell.com
Sodium Acetate Trihydrate 32318 Honeywell Research Chemicals From lab.honeywell.com
Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. The high boiling point of sodium allows the reactor to operate at ambient normal pressure but the drawbacks include its opacity which hinders visual maintenance and its explosive properties. As with any other substance however there are some precautions you should observe. Calcium chloride hydrate 6H2O 10g solid per pair. A sharp boiling point is an indication of the purity of the ester.
Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More.
Identification Product form. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. 0 360oC thermometers. This may take up to two hours. If a fluid consist of more than one component a solution components with. The ester we have prepared ethyl acetate ethyl ethanoate has the lowest boiling point of all the possible components in the mixture.
 Source: wikidata.org
Source: wikidata.org
If a fluid consist of more than one component a solution components with. Vapor or saturation pressure. Remove the flask from the heat and let it cool slowly without disturbing it. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. Vapor or saturation pressure depends on temperature.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. Saturated sodium carbonate solution 1 Bottle. The Ethyl Acetate that is collected is. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. At some point the Ethyl Acetate is going to get all distilled over.
It is widely used across a number of industrial sectors. When all Ethyl Acetate is distilled the temperature suddenly rises. Glycolic acid is found in some sugar-crops. Ethyl ethanoate ethyl acetate is an ester. Chemical Properties of Ethyl Acetate Few chemical properties of ethyl acetate are listed below Hydrolysis On reaction with water ethyl acetate gives ethanol and acetic acid.

The crystal structure of anhydrous Na 2 SO 3 is hexagonal whereas the heptahydrate crystals have a monoclinic structure. Sodium Sulfate Anhydrous CAS-No. Radioactive sodium-24 may be produced by neutron bombardment during operation posing a slight radiation hazard. Sodium sulfite does not have a specific boiling point since it tends to decompose at high temperatures. At some point the Ethyl Acetate is going to get all distilled over.
 Source: byjus.com
Source: byjus.com
Vapor or saturation pressure depends on temperature. Saturated sodium carbonate solution 1 Bottle. Sodium Sulfate Anhydrous CAS-No. The Food and Drug Administration FDA has stated that ethylene vinyl acetate is safe when used in food production packaging or transportation and its not an especially dangerous material. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each.
 Source: cameo.mfa.org
Source: cameo.mfa.org
Calcium chloride hydrate 6H2O 10g solid per pair. Gloves The Synthesis of Ethyl Ethanoate. Its boiling point is 771. Sodium sulfite does not have a specific boiling point since it tends to decompose at high temperatures. Naturally occurring in mammals phenylacetate induces differentiation growth inhibition and apoptosis in tumor cells.
 Source: lab.honeywell.com
Source: lab.honeywell.com
Naturally occurring in mammals phenylacetate induces differentiation growth inhibition and apoptosis in tumor cells. Try to keep the stream of the. Ethyl ethanoate ethyl acetate is an ester. The pressure exerted by the vapor phase is called the. It is soluble in water ethanol acetone etc.

58 Monday March 26 2012 Rules and Regulations Date of issue. Identification Product form. The vapor pressure of a liquid is defined as the pressure exerted by the molecules that escapes from the liquid to form a separate vapor phase above the liquid surface. The crystal structure of anhydrous Na 2 SO 3 is hexagonal whereas the heptahydrate crystals have a monoclinic structure. The Food and Drug Administration FDA has stated that ethylene vinyl acetate is safe when used in food production packaging or transportation and its not an especially dangerous material.
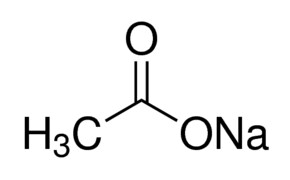 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Radioactive sodium-24 may be produced by neutron bombardment during operation posing a slight radiation hazard. 0 360oC thermometers. Its boiling point is 771. The Ethyl Acetate that is collected is. Saturated sodium carbonate solution 1 Bottle.

Its boiling point is 771. Naturally occurring in mammals phenylacetate induces differentiation growth inhibition and apoptosis in tumor cells. The ester we have prepared ethyl acetate ethyl ethanoate has the lowest boiling point of all the possible components in the mixture. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. Its boiling point is 771.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium acetate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.