Resorcinol boiling point
Home » datasheet » Resorcinol boiling pointResorcinol boiling point
Resorcinol Boiling Point. Only then can the molecule escape from the liquid into the gaseous state. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. The endpoint is the point at which the titration is complete as determined by an indicator. Many xanthene dyes can be prepared by condensation of derivates of phthalic anhydride with derivates of resorcinol or 3-aminophenol.
 What Is The Reason For The Boiling Point Of Benzene 1 4 Diol Being Higher Than That Of Benzene 1 3 Diol Chemistry Stack Exchange From chemistry.stackexchange.com
What Is The Reason For The Boiling Point Of Benzene 1 4 Diol Being Higher Than That Of Benzene 1 3 Diol Chemistry Stack Exchange From chemistry.stackexchange.com
In the classic strong acid-strong base titration the endpoint of a titration is. Sulfur S is an element that can never be overlooked. 101 to 102 C 214 to 216 F. And induces promyelocytic leukemia cell differentiation anti. This is ideally the same volume as the equivalence point. State at 20C.
Thereafter resorcinol formaldehyde RF resin was.
Many xanthene dyes can be prepared by condensation of derivates of phthalic anhydride with derivates of resorcinol or 3-aminophenol. The boiling point is a rough measure of the amount of energy necessary to separate a liquid molecule from its nearest neighbors. 310 to 312 C 590 to 594 F. Multiple Choice Questions are an important part of exams for Grade 12 Chemistry and if practiced. Reaction of neutral ferric chloride solution. He volume of added titrant at which the number of moles of titrant is equal to the number of moles of analyte or some multiple thereof as in polyprotic acids.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Since plastic resin glues dont stain and clean up easily mix with water and are a fraction of the cost of resorcinol theyre my choice in lieu of. 374 to 375 K Boiling point. The endpoint is the point at which the titration is complete as determined by an indicator. 33 S 34 S. 583 to 585 K Hazards R.

The MCQ Questions for Class 12 Chemistry with answers have been prepared as per the latest syllabus NCERT books and examination pattern suggested in Standard 12 by CBSE NCERT and KVS. After neutralization acetone is distilled off at normal pressure and resorcinol under vacuum. 1 H2SO4 in boiling acetone with a reaction time of 30 min. Production from shale oil. Alfa 10 was diluted in 100 ml water.
 Source: chemsynthesis.com
Source: chemsynthesis.com
583 to 585 K Hazards R. The page toward you and the vertical bonds point into the page away from you. Let the solution cool down. Photochemistry of the Xanthene Dyes. 374 to 375 K Boiling point.

Online API to Specific Gravity calculator. 583 to 585 K Hazards R. 310 to 312 C 590 to 594 F. Fischer projections are used to indicate the stereochemistry of each chiral carbon in the molecule and to compare monosaccharide structures easily. 4-Methylcatechol is an isomer found as its methyl ether in beech-wood tar.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The color of the precipitate gives a rough estimate of a reducing sugar present in the sample. Online API to Specific Gravity calculator. Figure 22 Intermolecular Hydrogen Bonding. The information mentioned in point g of paragraph 1 shall be expressed by using the common ingredient name set out in the glossary provided for in Article 33. The page toward you and the vertical bonds point into the page away from you.
 Source: researchgate.net
Source: researchgate.net
The cleavage of DHP to resorcinol and acetone is carried out under acid catalysis preferably with ca. MW 92 bp 111 C 2318 F. Further purification of resorcinol can be achieved by recrystallization or extraction. The endpoint is the point at which the titration is complete as determined by an indicator. Orcinol is also.
 Source: researchgate.net
Source: researchgate.net
The color of the precipitate gives a rough estimate of a reducing sugar present in the sample. In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. The anhydrides of aromatic 12-dicarboxylic acids on heating with resorcinol gives a dye fluorescein. Not to mention that there over 40 of said trendy shades to pick from and even some that will glow under a black light if you really want to get wild The color will last up to six weeks though keep in mind youll get the most vibrant results on hair thats either naturally light or has already. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights.
 Source: chemicalbook.com
Source: chemicalbook.com
In the absence of a common ingredient name a term as contained in a generally accepted nomenclature shall be used. API Gravity - API expresses the gravity or density of liquid petroleum products. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. A hydroxyl group is attached to an aromatic ring and it is strongly activating orthopara director phenols possess considerable reactivity at their ortho and para carbons toward electrophilic aromatic substitution. The boiling point is a rough measure of the amount of energy necessary to separate a liquid molecule from its nearest neighbors.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
374 to 375 K Boiling point. The information mentioned in point g of paragraph 1 shall be expressed by using the common ingredient name set out in the glossary provided for in Article 33. Definitions of the acid dissociation constant and pKa are given below the figures together with the definition of some classes of organic acids. Further purification of resorcinol can be achieved by recrystallization or extraction. Since plastic resin glues dont stain and clean up easily mix with water and are a fraction of the cost of resorcinol theyre my choice in lieu of.
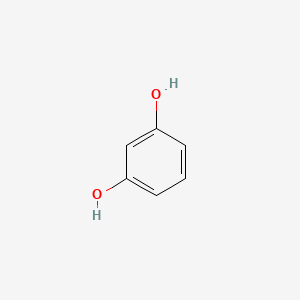
The cleavage of DHP to resorcinol and acetone is carried out under acid catalysis preferably with ca. 310 to 312 C 590 to 594 F. Electron configuration Ne 3s²3p⁴. 061514 Biochemistry For Medics- Lecture notes 15 Negative Reaction Positive Reaction 16. Only then can the molecule escape from the liquid into the gaseous state.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title resorcinol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.