Pure benzoic acid melting point range
Home » datasheet » Pure benzoic acid melting point rangePure benzoic acid melting point range
Pure Benzoic Acid Melting Point Range. Most pure organics melt over a narrow temperature range of 1-2ºC if heated slowly enough. Group 3 is assigned Esters A and C These assignments will allow some replicate samples in case there are experimental errors. 125 Medium-purity terephthalic acid typically has 250 ppm 4-formylbenzoic acid and acid. For example a pure.
 Measuring The Melting Points Of Compounds And Mixtures Odinity From odinity.com
Measuring The Melting Points Of Compounds And Mixtures Odinity From odinity.com
First the samples were packed into the melting point tube. The melting point range for pure solids is narrow usually only 1 to 2 degrees Celsius known as a sharp melting point. The apparatus used to measure melting points can be simple oil baths to hot-stage apparatus where the melting process is observed with the aid of a microscope. Of the melting range. Use vacuum filtration to isolate and dry. Kyselina p-nitrobenzoova Czech EINECS 200-526-2.
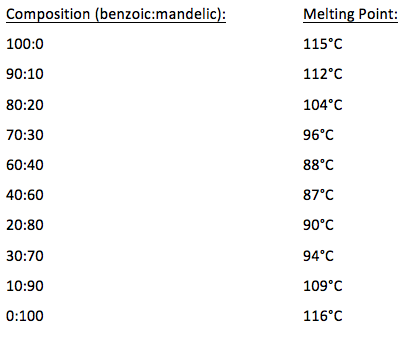
The purity of the samples will be indicated by their melting points ranges.
If the melting. There are three main reasons to record the melting point of a. This was accomplished by pressing the open end of the tube into the sample until the sample was about 2 mm into the tube. Glacial acetic acid and. For example a pure. A pure compound will melt over a relatively narrow temperature range impurities both lower and widen the temperature range over which a compound melts.
 Source: chegg.com
Source: chegg.com
Guaiacol ˈ ɡ w aɪ ə k ɒ l is a naturally-occurring organic compound with the formula C 6 H 4 OHOCH 3Although it is biosynthesized by a variety of organisms this aromatic oil is usually derived from guaiacum or wood creosoteIt is also found in essential oils from celery seeds tobacco leaves orange leaves and lemon peels. 4-Formylbenzoic acid ppm or 25. The purity of the samples will be indicated by their melting points ranges. Group 3 is assigned Esters A and C These assignments will allow some replicate samples in case there are experimental errors. The apparatus used to measure melting points can be simple oil baths to hot-stage apparatus where the melting process is observed with the aid of a microscope.
 Source: slideplayer.com
Source: slideplayer.com
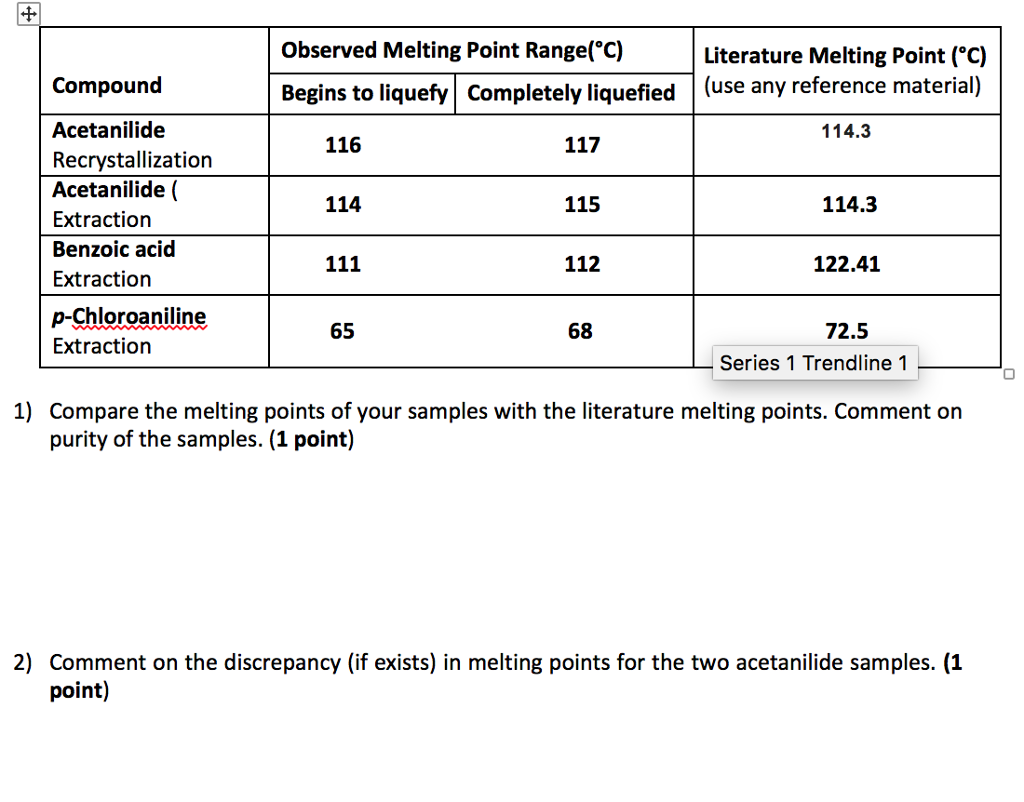
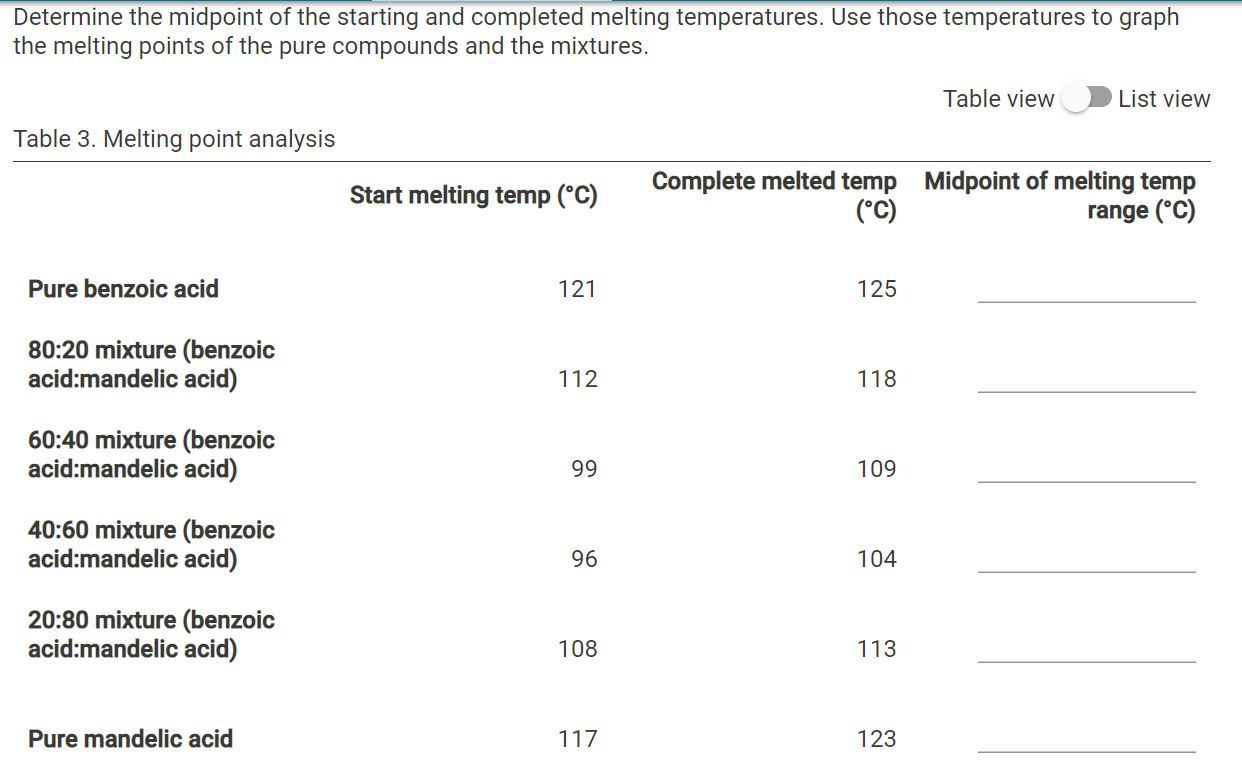
125 plus or minus 45. The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. Less pure substances will have a lower melting point and a larger melting range. You will then assess its purity by taking a melting point. An unknown sample of benzoic acid 2-naphthol and naphthalene Table 1 was massed.
 Source: chegg.com
Source: chegg.com
This online resource looks at the harmful effects of acid rain. How solar panels work 0. Benzoic acid solid and 1-Propanol liquid Ester C. Find the melting point range of the pure unknown substance by first quickly determining an approximate melting range on a fast ramp 20 oCmin from 70-210 oC and then doing a slow careful melting range with the second capillary tube you prepared use a ramp of 2 oCmin and start about 15 oC below the. 92 g The real laboratory that we will do is the recrystallization of benzoic acid from water with the temperature gradient method and the pH method.
 Source: researchgate.net
Source: researchgate.net
Impurities cause structural defects that make the intermolecular interactions between the molecules easier to overcome. Acetic acid liquid and 3-Methyl-1-butanol liquid Group 1 is assigned Esters A and B Group 2 is assigned Esters B and C Circle the 2 esters your group has been assigned. Its main constituents are. Impurities cause structural defects that make the intermolecular interactions between the molecules easier to overcome. A sharp melting point is often evidence that a sample is fairly pure and a wide melting range is evidence that it is not pure.
 Source: chegg.com
Source: chegg.com
The colors are light yellow medium yellow or dark brown and white. Melting point range for your unknown compound 3 the compound names and melting ranges for your mixture melting experiments and 4 the name structure and melting point range of the known compound that most clearly agrees with your measured melting point. Insert the capillary tubes containing your recrystallized benzoic acid along with the tube containing pure benzoic acid into the melt point apparatus. Turn the temperature control knob to about the 4-5 setting. This online resource describes how photovoltaic cells work.

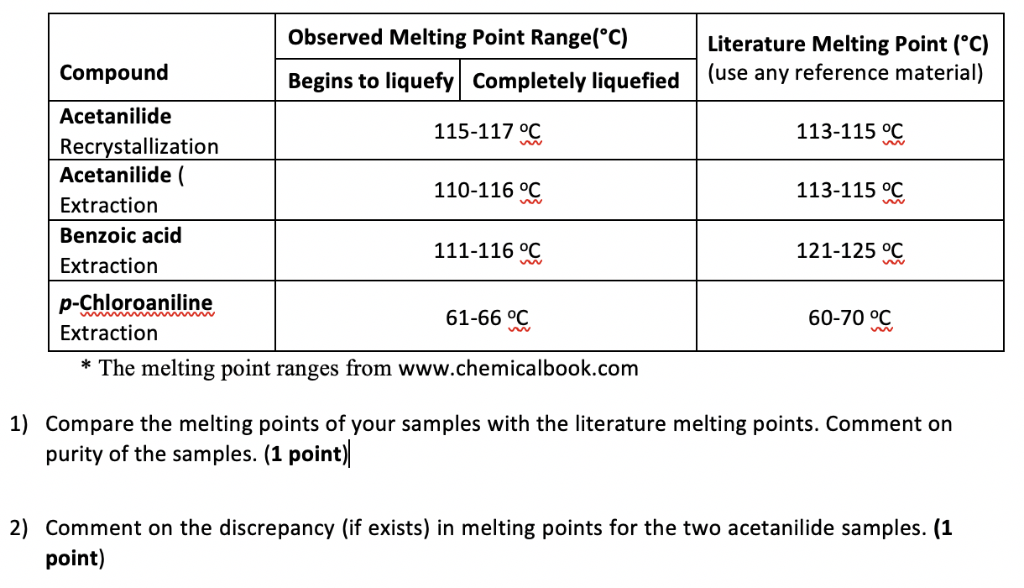
The presence of a soluble impurity almost always causes a decrease in the melting point expected for the pure compound and a broadening of the. If benzoic were contaminated with an impurity the melting point range might decrease and broaden to 117-120 C. Be sure to grind each sample well before introducing it into the melting point tube you may use a glass rod and a watch glass. A pure compound will melt over a relatively narrow temperature range impurities both lower and widen the temperature range over which a compound melts. For example pure benzoic acid has a melting point range of 121-123 C.
 Source: odinity.com
Source: odinity.com
The melting point range of 5 o C indicates that the substance is impure. The temperature at which all material of a contaminated substance is molten is usually lower than that of a pure substance. For example if A is cinnamic acid mp. If the compound melts over a very narrow range it can usually be assumed that the compound is relatively pure. The melting point range of 5 o C indicates that the substance is impure.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Latest Questions View All Sign in to. Guaiacol ˈ ɡ w aɪ ə k ɒ l is a naturally-occurring organic compound with the formula C 6 H 4 OHOCH 3Although it is biosynthesized by a variety of organisms this aromatic oil is usually derived from guaiacum or wood creosoteIt is also found in essential oils from celery seeds tobacco leaves orange leaves and lemon peels. The lowest mixture melting point e is called the eutectic point. If the melting. Kyselina p-nitrobenzoova Czech EINECS 200-526-2.
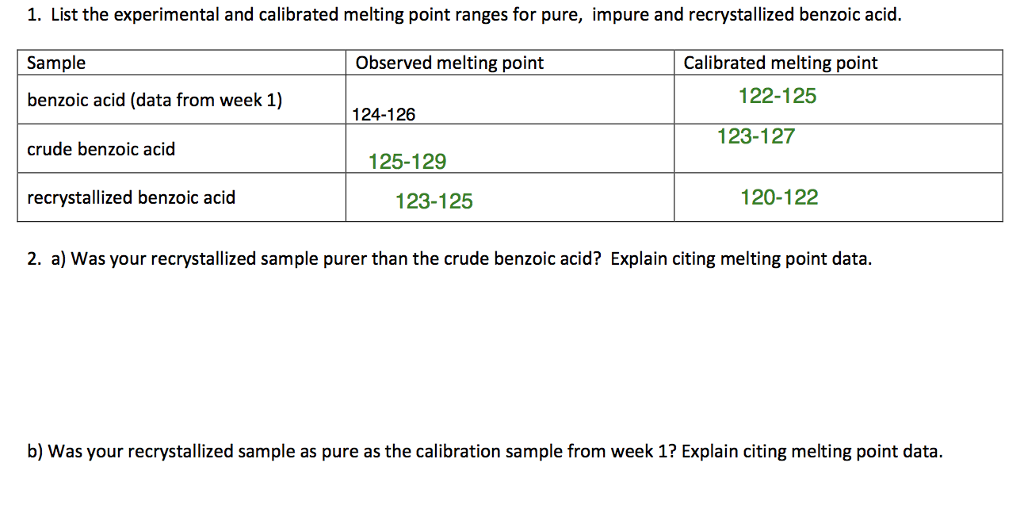 Source: chegg.com
Source: chegg.com
Coarse to fit into the capillary tube. If benzoic were contaminated with an impurity the melting point range might decrease and broaden to 117-120 C. The melting point of benzoic acid is approximately 122 C 2002 Gilbert and Martin CD-Rom MSDS Data. Too large of an amount of solid will result in a larger melting point range and should be avoided. Higher concentrations to obtain the same results that pure sorbic acid provides.
 Source: researchgate.net
Source: researchgate.net
This online resource looks at the harmful effects of acid rain. Most pure organic compounds melt over a narrow temperature range of 1-2 C. The unknown sample may not be separated using melting points because both benzoic acid and 2-naphthol have a melting point of 123C. Kyselina p-nitrobenzoova Czech EINECS 200-526-2. Benzoic acid solid and 1-Propanol liquid Ester C.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title pure benzoic acid melting point range by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.