Propyne boiling point
Home » datasheet » Propyne boiling pointPropyne boiling point
Propyne Boiling Point. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. A A crystalline hard structure b A low melting point and low boiling point c An ionic bond d A strong force of attraction between its molecules. Melting point of ice to give water. Using Reppe chemistry methyl acetylene is converted to MMA.
 1 Propyne 3 3 3 D3 9ci 13025 73 5 Wiki From guidechem.com
1 Propyne 3 3 3 D3 9ci 13025 73 5 Wiki From guidechem.com
The following reaction is an example of a 4NH 3 g 50 2 g à 4NO g 6 H 2 O g Displacement Reaction. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. Boiling point of alkanes decreases on branching. Which alkyl halide from the following pairs would you expect to react more rapidly by an S N 2 mechanism. CHCCH 3 CO CH 3 OH CH 2 CCH 3CO 2 CH 3. Academiaedu is a platform for academics to share research papers.
Propyne exists in equilibrium with propadiene the mixture of propyne and propadiene being called MAPD.
N-octane iso-octane 2 2 3 3-tetramethyl butane iii. CH 3 2 CHCl isopropylchloride or 2-chloropropane ClCH 2 CH 2 CH 3 1-chloropropane ClCH 2 CH 2 CH 2 CH 3 1-chlorobutane 107. But when you want to use a strong bulky poorly-nucleophilic base potassium t. Propargyl bromide C3H3Br CID 7842 - structure chemical names physical and chemical properties classification patents literature biological activities. 2 to 0 decrease in oxidation number is reduction AND increase in oxidation number is. Alkanes with even number of carbon atoms have higher melting points as compared to next higher or lower alkanes with odd number of carbon atoms.
 Source: guidechem.com
Source: guidechem.com
CH2CH-CH3 propyne CH3CCH trans-2-butene CH3-CHCH-CH3 undecane C11H24 112-trimethylcyclohexane C9H18 113-trimethylcyclopentane C8H16 114-trimethylcyclohexane C9H18 11-dimethylcyclohexane C8H16 11-dimethylcyclopentane C7H14 1234-tetrahydronaphthalene C10H12 1245-tetramethylbenzene. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. Low boiling point high flammability high melting point. The increasing order of boiling point is. Alkanes with even number of carbon atoms have higher melting points as compared to next higher or lower alkanes with odd number of carbon atoms.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
BP VAF van der Waals forces VAF molecular mass or VAF SA surface area So boiling point order can be given as. 2 to 0 decrease in oxidation number is reduction AND increase in oxidation number is. PropsSI fluid pcrit Massic volume in m3kg is the inverse of density or volumic mass in kgm3. The concentrations of propyne in the propylene and butyne in the 13-butadiene flows dramatically decreased to be about 18 and 172 ppm respectively. These vapours of evaporating crude oil condense at different.

The molecular mass of 2-Methylbutane. The carbon atoms in saturated hydrocarbons _____. PropsSI D P 1e5 Q 0 fluid Same for saturated vapor vG. Low boiling point high flammability high melting point. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Boiling point increases with increase in molecular mass. The surrounding temperature around a storage tank should always. Calculate the number of grams of an impurity of M100 gmole which be required to raise the boiling point of 50 grams of chloroform by this amount A. Low boiling point high flammability high melting point. CH2CH-CH3 propyne CH3CCH trans-2-butene CH3-CHCH-CH3 undecane C11H24 112-trimethylcyclohexane C9H18 113-trimethylcyclopentane C8H16 114-trimethylcyclohexane C9H18 11-dimethylcyclohexane C8H16 11-dimethylcyclopentane C7H14 1234-tetrahydronaphthalene C10H12 1245-tetramethylbenzene.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Lets compute the massic volume of liquid at 1bar 1e5 Pa of pressure vL 1 CP. Low boiling point high flammability high melting point. Manish Bhojasia a technology veteran with 20 years Cisco Wipro is Founder and CTO at SanfoundryHe is Linux Kernel Developer SAN Architect and is passionate about competency developments in these areas. CHCCH 3 CO CH 3 OH CH 2 CCH 3CO 2 CH 3. Melting point of ice to give water.
Source: chemspider.com
A compound X consists of only molecules. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. When the vapours evaporate and meet a liquid fraction whose temperature is below the boiling point of vapor it partly condenses. Have only single bonds. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher.

72 g mol-1 The molecular mass of 2-Methylbutane. Boiling point increases with increase in molecular mass. Have only single bonds. Reacts as either a liquid or gas with alcohols explosion molten aluminum explosion silane explosion bromine pentafluoride carbon disulfide explosion catalyzed by iron 1-chloro-2-propyne excess chlorine causes an explosion dibutyl phthalate explosion at 118 C diethyl ether ignition diethyl zinc ignition glycerol explosion at 70-80. 72 g mol-1 The molecular mass of 2-Methylbutane.
 Source: tcichemicals.com
Source: tcichemicals.com
When the vapours evaporate and meet a liquid fraction whose temperature is below the boiling point of vapor it partly condenses. 2 to 1 oxygen. The carbon atoms in saturated hydrocarbons _____. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. Lets compute the massic volume of liquid at 1bar 1e5 Pa of pressure vL 1 CP.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
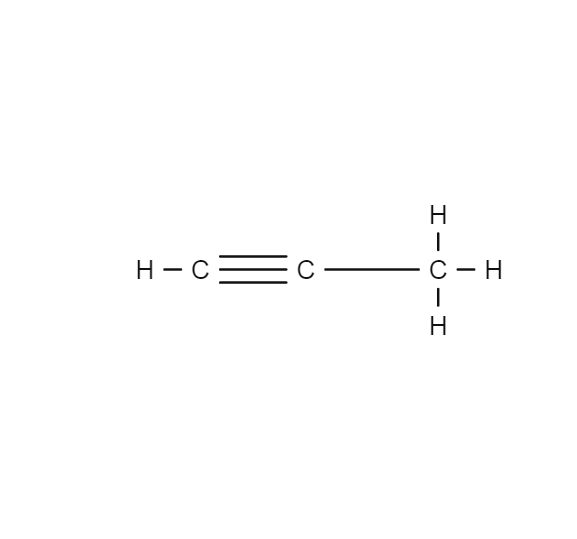
Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. Low boiling point high flammability high melting point. Propyne CH3CCH or CH3-CCH or C3H4 CID 6335 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. A A crystalline hard structure b A low melting point and low boiling point c An ionic bond d A strong force of attraction between its molecules.
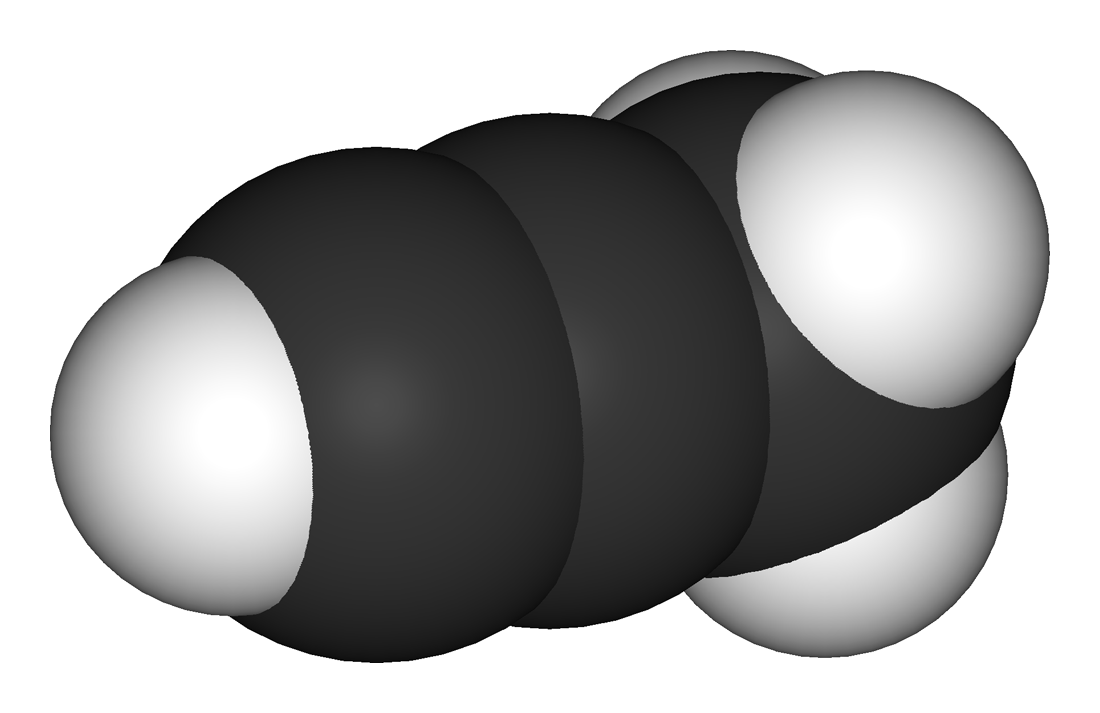 Source: en.wikipedia.org
Source: en.wikipedia.org
Combustion of Liquified Petroleum GasLPG Answer. Neutralisation Reaction 1 and 4. PropsSI fluid pcrit Massic volume in m3kg is the inverse of density or volumic mass in kgm3. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. Combustion of Liquified Petroleum GasLPG Answer.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title propyne boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.