Propylene boiling point
Home » datasheet » Propylene boiling pointPropylene boiling point
Propylene Boiling Point. Gas density at boiling point. Heat capacity at constant pressure Cp. KJkgK 3487E-1 BTUlbF 1458966 JkgK 3487E-1 kcalkgK. Solution We can solve this problem using four steps.

Melting PointRange-60 C -76 F Boiling PointRange 187 C 3686 F Flash Point 99 C 2102 F Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 126 vol Lower 26 vol Vapor Pressure 013 mbar 20 C Vapor Density 262 Air 10 Specific Gravity 103 - 104 Solubility Soluble in water Partition. At the melting point the solid and liquid phases exist in equilibrium. Page 1 For Divisions 11 12 13 or 15 Use GUIDE 112 For Divisions 14 or 16 Use GUIDE 114 Do you see an explosiv e placa rd o label. 0366 D gas Hazards Safety data sheet. Vapors irritate eyes skin and respiratory system. Magnetic susceptibility χ-31510 6 cm 3 mol Viscosity.
Refer to NFPA Code 13 for.
The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. Propylene glycol IUPAC name. Refer to NFPA Code 13 for. 0366 D gas Hazards Safety data sheet. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Solution We can solve this problem using four steps.
 Source: thermopedia.com
Source: thermopedia.com
Fuel Boiling Point o F Acetaldehyde. Fuel Boiling Point o F Acetaldehyde. Propylene oxide appears as a clear colorless volatile liquid with an ethereal odor. However aqueous solutions of propylene glycol greater than 22 by weight if heated sufficiently will produce flammable vapors. Heat capacity at constant pressure Cp.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Vapors irritate eyes skin and respiratory system. EU classification DSD outdated R-phrases outdated 12 S-phrases outdated 9-16-33 NFPA 704 fire diamond 1. 0366 D gas Hazards Safety data sheet. Melting PointRange-60 C -76 F Boiling PointRange 187 C 3686 F Flash Point 99 C 2102 F Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 126 vol Lower 26 vol Vapor Pressure 013 mbar 20 C Vapor Density 262 Air 10 Specific Gravity 103 - 104 Solubility Soluble in water Partition. KJkgK 52128E-1 BTUlbF 2181014 JkgK 52128E-1 kcalkgK.
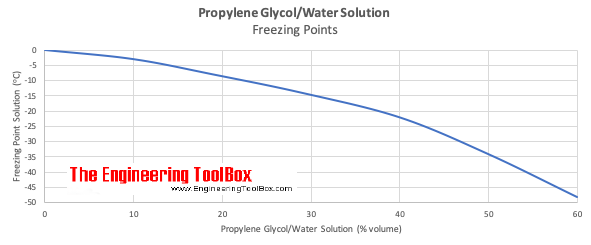 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
A substances melting point depends on pressure and is usually specified at standard pressure in reference materials. At the melting point the solid and liquid phases exist in equilibrium. Propylene glycol IUPAC name. Propylene glycol by weight have no flash point as obtained by standard test methods. KJkgK 52128E-1 BTUlbF 2181014 JkgK 52128E-1 kcalkgK.
 Source: researchgate.net
Source: researchgate.net
Some fuels and their boiling points at atmospheric pressure. Some fuels and their boiling points at atmospheric pressure. All boiling points below are normalatmospheric boiling points. Vapors irritate eyes skin and respiratory system. 476 C 537 F.
 Source: researchgate.net
Source: researchgate.net
Propylene oxide appears as a clear colorless volatile liquid with an ethereal odor. Containing two alcohol groups it is classed as a diolIt is miscible with a broad range of solvents including water acetone and chloroformIn general glycols are non-irritating and have very low volatility. KJkgK 3487E-1 BTUlbF 1458966 JkgK 3487E-1 kcalkgK. Page 1 For Divisions 11 12 13 or 15 Use GUIDE 112 For Divisions 14 or 16 Use GUIDE 114 Do you see an explosiv e placa rd o label. Propylene glycol IUPAC name.

Always drain and flush systems containing propylene glycol with water before welding or other maintenance. However aqueous solutions of propylene glycol greater than 22 by weight if heated sufficiently will produce flammable vapors. 476 C 537 F. Some fuels and their boiling points at atmospheric pressure. Magnetic susceptibility χ-31510 6 cm 3 mol Viscosity.
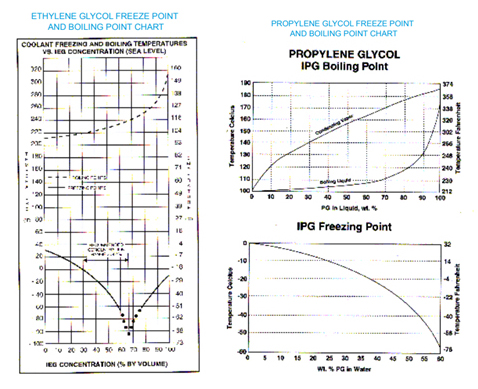 Source: penray.com
Source: penray.com
Propylene glycol by weight have no flash point as obtained by standard test methods. Propylene Glycol Solution by mass. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Propylene glycol by weight have no flash point as obtained by standard test methods. Propylene oxide appears as a clear colorless volatile liquid with an ethereal odor.
 Source: researchgate.net
Source: researchgate.net
Magnetic susceptibility χ-31510 6 cm 3 mol Viscosity. Always drain and flush systems containing propylene glycol with water before welding or other maintenance. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. KJkgK 3487E-1 BTUlbF 1458966 JkgK 3487E-1 kcalkgK. The melting point is also referred to as liquefaction point solidus or liquidus.
 Source: researchgate.net
Source: researchgate.net
They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Liver 3000 pmolg DNA. Magnetic susceptibility χ-31510 6 cm 3 mol Viscosity. The melting point of a substance is the temperature at which it changes state from solid to liquid at atmospheric pressure. 834 µPas at 167 C Structure Dipole moment.

Propylene glycol by weight have no flash point as obtained by standard test methods. Flammable over a wide range of vapor-air concentrations. Heat capacity at constant pressure Cp. All boiling points below are normalatmospheric boiling points. KJkgK 3487E-1 BTUlbF 1458966 JkgK 3487E-1 kcalkgK.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title propylene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.