Propene boiling point
Home » datasheet » Propene boiling pointPropene boiling point
Propene Boiling Point. Interactive problems to aid students of organic chemistry. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. National Toxicology Program Chemical Repository Database. It is an impurity in some.
 Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
Why Do Cyclic Hydrocarbons Have Higher Boiling Points Than Their Acyclic Isomers Chemistry Stack Exchange From chemistry.stackexchange.com
-1404 deg C BP exp database. Centre number Candidate number. Research Triangle Park North Carolina. H Please write clearly in block capitals. The boiling point of each alkene is very similar to that of the alkane with the same number of carbon atoms. 2655 0K ความถวงจาเพาะ Specific Gravity 334 0C ของเหลว.
Propene is a product of combustion from forest fires cigarette smoke and motor vehicle and aircraft exhaust.
24 C 11 F. Check the calculator against the following values. In all calculations show clearly how you work out your answer. New Window-539 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. It is an impurity in some. -1404 deg C BP exp database.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Vapour pressure at. -1404 deg C BP exp database. At the approximate boiling point of the desired aldehyde and not higher. Z Factor at critical point 365 K 46 MPa 0275. In all calculations show clearly how you work out your answer.
 Source: youtube.com
Source: youtube.com
Boiling Pt deg C. AH 2O bH 2S cH 2Se dH 2Te 15. Which of the following statement is correct. Alcohol - ethyl grain ethanol C. Their boiling points increase with increasing molar mass and they are insoluble in water.
 Source:
Source:
Key Takeaway The physical properties of alkenes are much like those of the alkanes. -13088 Mean or Weighted MP VPmm Hg25 deg C. Check the calculator against the following values. Which of the following statement is correct. Temperature K A B C Reference Comment.
 Source: thermopedia.com
Source: thermopedia.com
Their boiling points increase with increasing molar mass and they are insoluble in water. 2 SPECIMEN MATERIAL 0 1 This question is about organic compounds. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. H Please write clearly in block capitals. Check the calculator against the following values.
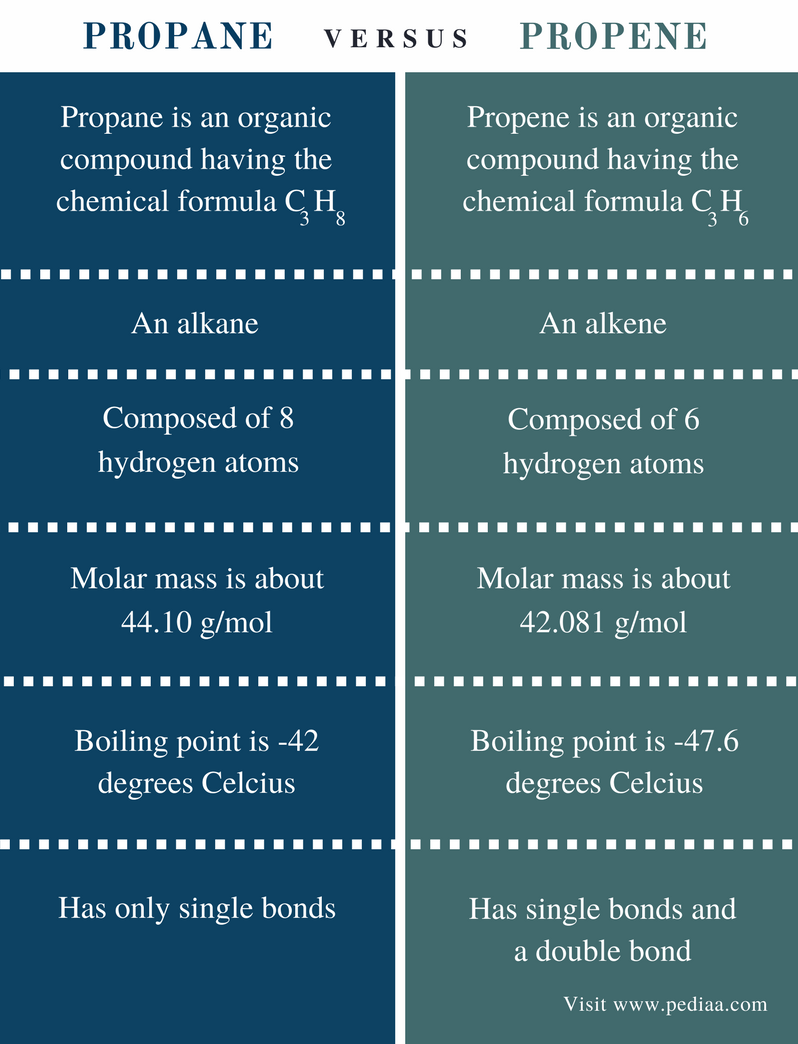 Source: pediaa.com
Source: pediaa.com
Vapour pressure at. 165 to 169 C 329 to 336 F. Boiling point increases with increase in the number of carbon atoms because of increase in molecular mass. Forenames Candidate signature. 438 to 442 K.
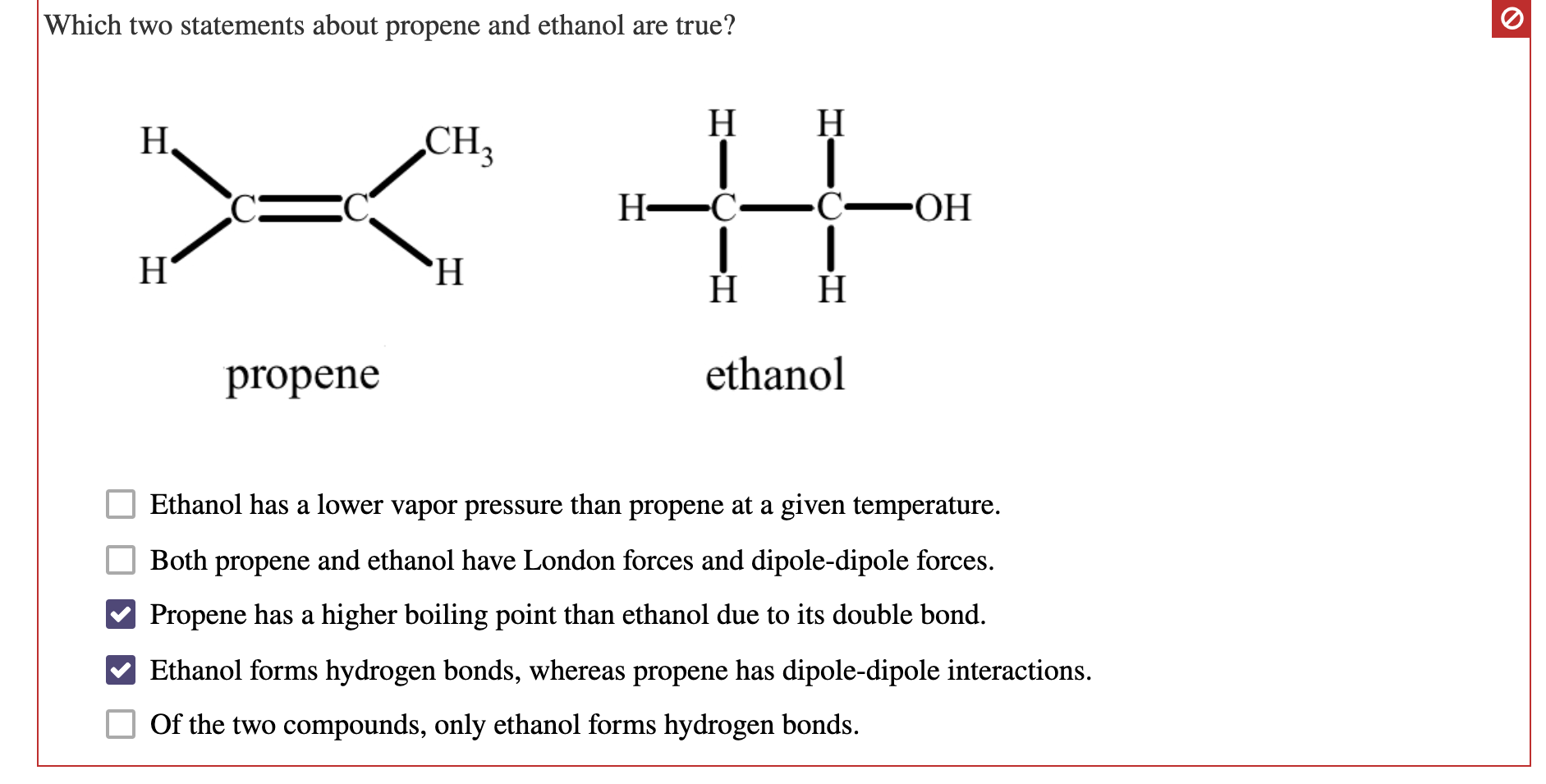 Source: chegg.com
Source: chegg.com
จดเดอด Boiling Point 1 atm. Propene is a product of combustion from forest fires cigarette smoke and motor vehicle and aircraft exhaust. -1404 deg C BP exp database. Further among isomeric alcohols 1 alcohols have higher boiling points than 2 alcohols because of higher surface area in 1 alcohols. C 9 H 10.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Acetone CH 3 COCH 3. Ethene and Propene are the first two hydrocarbons. 2 C 3 H 6 9 O 2 6 CO 2 6 H 2 O Environmental safety. Forenames Candidate signature. Acetic acid anhydride CH 3 COO 2 O.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Ethene and Propene are the first two hydrocarbons. At the approximate boiling point of the desired aldehyde and not higher. -1404 deg C BP exp database. Calculate propene or propylene gas molar volume compressibility factor Z density and vapour pressure from critical point constants using the Peng Robinson Soave Redlich Kwong and Van Der Waals equations of state EOS. Research Triangle Park North Carolina.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
165 to 169 C 329 to 336 F. Forenames Candidate signature. Research Triangle Park North Carolina. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Same chemical properties.

165 to 169 C 329 to 336 F. 2 C 3 H 6 9 O 2 6 CO 2 6 H 2 O Environmental safety. 223E003 Mean VP of Antoine Grain methods MP exp database. 1995 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN v142. C 9 H 10.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title propene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.