Propanoic acid boiling point
Home » datasheet » Propanoic acid boiling pointPropanoic acid boiling point
Propanoic Acid Boiling Point. Propyl propionate is a propanoate ester resulting from the formal condensation of the hydroxy group of propanol with the carboxy group of propanoic acid. Propionic acid p r oʊ p i ˈ ɒ n ɪ k from the Greek words protos meaning first and pion meaning fat. It is a conjugate acid of a pyruvate. The physical differences observed between a fat like butter and an oil like sunflower oil are due to differences in melting points of the mixture of esters they contain.
The physical differences observed between a fat like butter and an oil like sunflower oil are due to differences in melting points of the mixture of esters they contain. It is produced on a large scale from benzene as a precursor to anilineIn the laboratory it is occasionally used as a solvent especially for electrophilic reagents. Propanoic acid ethyl ester Molecular Formula. Predicting Physical Properties Which member of each of the following pairs of compounds would you expect to have a higher boiling point. Ethanol and ethanoic acid. Protus prion propanoic acid-208 ºC.
In asymmetrical acid anhydride two different carboxylic acids are used like the dehydration of benzoic acid and propanoic acid so the prefix is benzoic propanoic and anhydride is a suffix.
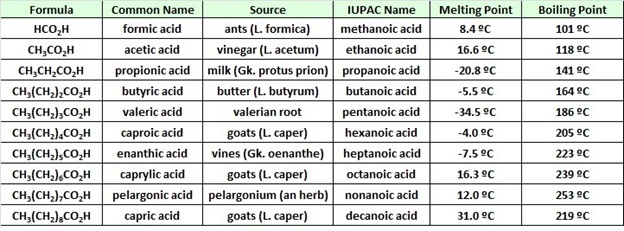
Propanoic acid propyl ester. It is a liquid with a pungent and unpleasant smell somewhat resembling body odorThe anion CH 3 CH 2 CO 2 as well as the salts and esters of propionic acid are. CH 3 CH 2 2 CO 2 H. Butyrum butanoic acid-55 ºC. CH 3 CH 2 3 CO 2 H. For example the compound CH 3 CH 2 COOH has three carbon atoms and is called propanoic acid from propane the name for a three-carbon chain with -oic acid the suffix for this class of compounds appended.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Pyruvic acid is a 2-oxo monocarboxylic acid that is the 2-keto derivative of propionic acid. Boiling Point C Feature. It derives from. It was first prepared by French chemist Charles Frederic Gerhardt in 1852 through heating potassium. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol ethyldimethylamine 22 Examples.
 Source: chem.libretexts.org
Source: chem.libretexts.org
CH 3 CH 2 3 CO 2 H. Also known as propanoic acid is a naturally occurring carboxylic acid with chemical formula CH 3 CH 2 CO 2 H. 2 Table 1 shows the boiling point flammability and viscosity of C 18H 38 compared with the other hydrocarbons shown in the equation. Boiling Point C Feature. 15687-27-1 RN 2-4-Isobutylphenylpropanoic acid ACDIUPAC Name.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. It is a metabolite obtained during glycolysis. Propionic acid p r oʊ p i ˈ ɒ n ɪ k from the Greek words protos meaning first and pion meaning fat. 2-aminopropane or 2-aminohexane. Now take a alcohol that is a shorter chain such as propanol.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
It derives from. It was first prepared by French chemist Charles Frederic Gerhardt in 1852 through heating potassium. Melting Point C Physical Form. It has a role as a human metabolite a rat metabolite a biomarker and a fungal metabolite. It is produced on a large scale from benzene as a precursor to anilineIn the laboratory it is occasionally used as a solvent especially for electrophilic reagents.
 Source: chegg.com
Source: chegg.com
ΔH trs kJmol Temperature K Initial Phase Final Phase Reference Comment. The Ka value of HCO_3- is determined to be 50E-10. Propyl propionate is a propanoate ester resulting from the formal condensation of the hydroxy group of propanol with the carboxy group of propanoic acid. A dimeric species shown on the right held together by two hydrogen bonds is a major component of the liquid state. CH 3 CH 2 2 CO 2 H.
Source: chemspider.com
For example pentanoic acid the most. The physical differences observed between a fat like butter and an oil like sunflower oil are due to differences in melting points of the mixture of esters they contain. 157 C 4 mm 3648476 C 760 mmHg Alfa Aesar B20989. Notice that the acid is named by counting up the total number of carbon atoms in the chain - including the one in the -COOH groupSo for example CH 3 CH 2 COOH is propanoic acid and CH 3 CH 2 COO is the propanoate group. Boiling Point C Feature.

Protus prion propanoic acid-208 ºC. The physical differences observed between a fat like butter and an oil like sunflower oil are due to differences in melting points of the mixture of esters they contain. Acetic acid the ninth entry is an interesting case. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol ethyldimethylamine 22 Examples. Also known as propanoic acid is a naturally occurring carboxylic acid with chemical formula CH 3 CH 2 CO 2 H.
 Source: docbrown.info
Source: docbrown.info
Propanoic acid ethyl ester Molecular Formula. It has a role as a fundamental metabolite and a cofactor. CH 3 CH 2 2 CO 2 H. Predicting Physical Properties Which member of each of the following pairs of compounds would you expect to have a higher boiling point. Boiling point C.
 Source: sites.google.com
Source: sites.google.com
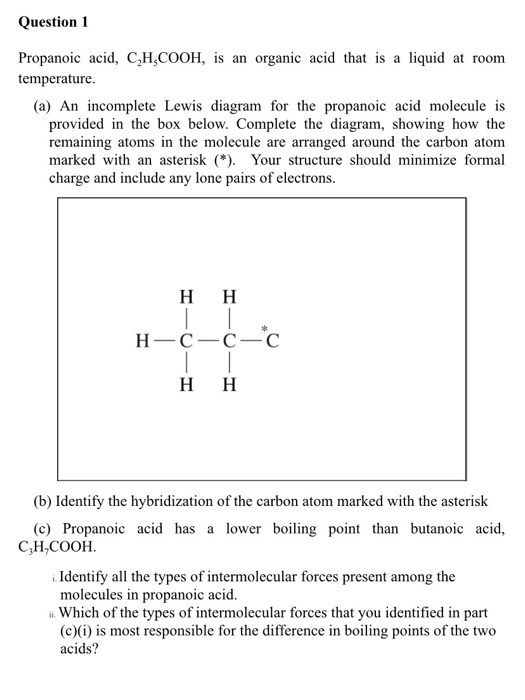
It derives from. Martin and Andon 1982 2. In a preparation of propanal propan-1-ol is added dropwise to the oxidising agent and the aldehyde is separated from the reaction mixture by distillation. Explain with reference to intermolecular forces why distillation allows propanal to be separated from the other organic compounds in this reaction. It derives from a propionic acid.
 Source: chemsynthesis.com
Source: chemsynthesis.com
If this is an accurate representation of the composition of this compound then we would expect its boiling point. But the propanol molecule is a much shorter chain so it has a smaller surface area than. Boiling point C. Nitrobenzene is an organic compound with the chemical formula C 6 H 5 NO 2It is a water-insoluble pale yellow oil with an almond-like odorIt freezes to give greenish-yellow crystals. A dimeric species shown on the right held together by two hydrogen bonds is a major component of the liquid state.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title propanoic acid boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.