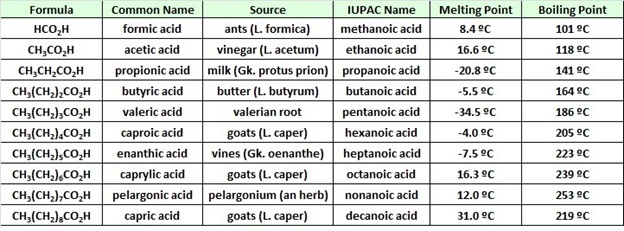
Propanoic acid boil point
Home » datasheet » Propanoic acid boil pointPropanoic acid boil point
Propanoic Acid Boil Point. A 218 L sample of butyric acid a substance present in rancid butter has a mass of 2088 g. Phenol and benzoic acid may be. 45359 g 1 ft gmL 6922 lbft 1 lb 3048 cm 3. 1 mL 11088gmL.
 Data Table Of Carboxylic Acids Boiling Point Trend Melting Points Solubility In Water Hydrogen Bonding Dimer Odours Smells Advanced A Level Organic Chemistry Revision Notes Doc Brown From docbrown.info
Data Table Of Carboxylic Acids Boiling Point Trend Melting Points Solubility In Water Hydrogen Bonding Dimer Odours Smells Advanced A Level Organic Chemistry Revision Notes Doc Brown From docbrown.info
1 mL 11088gmL. As hydrochloric acid 10 gL HCl the solution is prepared by. Boric oxide 215-125-8 1303-86-2 Repr. Since the density closely matches the known value we conclude that this is an authentic sample of ethylene glycol. What is the pH at the equivalence point. PDF Organic Chemistry by David Klein pdf download 3rd.
A 152 L sample of chloroform at 20 C has a mass of 2254 kg.
A 218 L sample of butyric acid a substance present in rancid butter has a mass of 2088 g. A It is a stronger acid than CH 3 COOH b It forms formyl chloride with PCl5 c It gives CO and H2 O on heating with conc. ΔH trs kJmol Temperature K Initial Phase Final Phase Reference Comment. None of the above. Add 10 drops of ethanol or other alcohol to the mixture. Boric acid 1 boric acid 2 233-139-2 1 234-343-4 2 10043-35-3 1 11113-50-1 2 Repr.
 Source: sites.google.com
Source: sites.google.com
When a substance in the liquid state is subjected to cooling heat is. Add 10 drops of ethanol or other alcohol to the mixture. Since the density closely matches the known value we conclude that this is an authentic sample of ethylene glycol. For propanoic acid hydrogen bonds form between the carbonyl group on one acid and the hydroxyl group on another. Dont conduct electricity - have no mobile ions or electrons except for graphite Strength.

None of the above. The process of conversion of a liquid state to a solid state is called freezing. In a ground-glass-stoppered flask and boil under a reflux condenser for 1 h. Equal to 7 c. When a substance in the liquid state is subjected to cooling heat is.
 Source: chem.libretexts.org
Source: chem.libretexts.org
And b the temperature of absolute zero in A. Ap chemistry chapter 4 practice test. In a ground-glass-stoppered flask and boil under a reflux condenser for 1 h. A 218 L sample of butyric acid a substance present in rancid butter has a mass of 2088 g. A 152 L sample of chloroform at 20 C has a mass of 2254 kg.
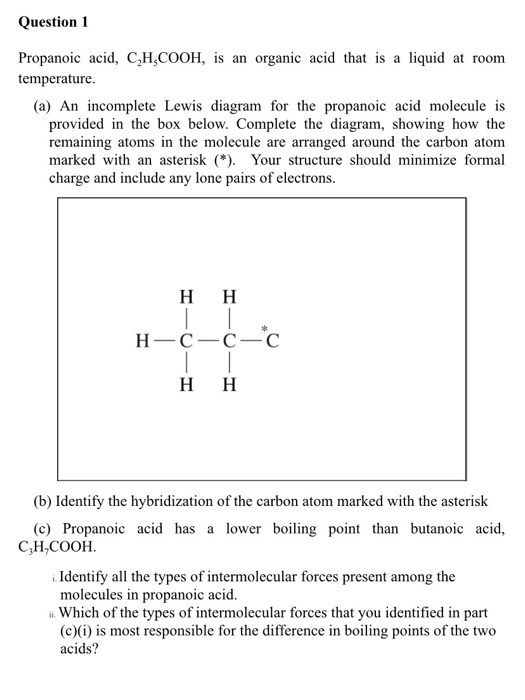 Source: chegg.com
Source: chegg.com
ΔH trs kJmol Temperature K Initial Phase Final Phase Reference Comment. As hydrochloric acid 10 gL HCl the solution is prepared by. Calculate the number of millilitres of1 M sodium hydroxiderequired for 1 g n1. Stand for 1 minute in the. Since the density closely matches the known value we conclude that this is an authentic sample of ethylene glycol.
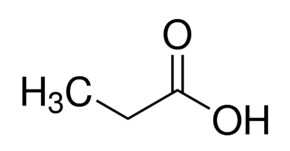 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Acidity of Carboxylic Acids 18 Carboxylic acids may to dissociate to a carboxylate anion and a hydrogen ion proton. C 55 ATP01ATP01corr 005-008-00-8 diboron trioxide. 1 Aquatic Acute 1 Aquatic Chronic 1 H317. Mass of one atom of an element relative to one twelfth of the mass of one atom. Less than 7 b.
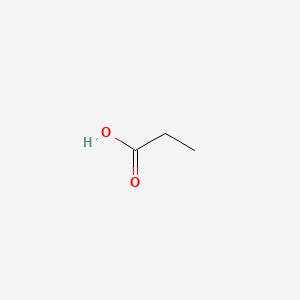
1 mL 11088gmL. C 55 ATP01ATP01corr 005-008-00-8 diboron trioxide. Enter the email address you signed up with and well email you a reset link. What would be a the boiling point of water in A. Thank you for your participation.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Add 10 drops of ethanol or other alcohol to the mixture. Add 10 drops of ethanol or other alcohol to the mixture. Which of the following statements is not true about HCOOH. Ap chemistry chapter 4 practice test. Carboxylic acids boil at considerably higher temperatures than alcohols ketones or aldehydes of similar molecular weight The high boiling point of carboxylic acids is attributed to their capacity to readily form stable hydrogen-bonded dimers.

Substituent Effects on the. C 31 005-009-00-3 tetrabutylammonium butyltriphenylborate 418-080-4 120307-06-4 Skin Sens. It is the opposite process of melting. This means that each molecule of propanoic acid can be part of two hydrogen bonds this is called dimerisation see figure below and so the boiling point is. Hard - exists in tetrahedral structure but graphite is soft 36 Metallic Bonding 44 Masses Relative atomic mass Ar.
 Source: docbrown.info
Source: docbrown.info
Phenol and benzoic acid may be. Heat the beaker gently on a tripod and gauze until the water begins to boil then stop heating. A 152 L sample of chloroform at 20 C has a mass of 2254 kg. Calculate the number of millilitres of1 M sodium hydroxiderequired for 1 g n1. For propanoic acid hydrogen bonds form between the carbonyl group on one acid and the hydroxyl group on another.
 Source: docbrown.info
Source: docbrown.info
Add 10 drops of ethanol or other alcohol to the mixture. Boric oxide 215-125-8 1303-86-2 Repr. Heat the beaker gently on a tripod and gauze until the water begins to boil then stop heating. Boric acid 1 boric acid 2 233-139-2 1 234-343-4 2 10043-35-3 1 11113-50-1 2 Repr. When a substance in the liquid state is subjected to cooling heat is.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title propanoic acid boil point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.