Propane boiling point
Home » datasheet » Propane boiling pointPropane boiling point
Propane Boiling Point. In contrast propane boils at -42C becoming gas vapour. C 30 H 62. Methane ethane propane butane etc. Propane is the principal ingredient of bottled gas particularly in northern states whereas butane with its considerably higher boiling and freezing points is more widely used in warmer southern states.
 Melting Point Trend For Alkanes Youtube From youtube.com
Melting Point Trend For Alkanes Youtube From youtube.com
C 8 H 18-57. It also shows the saturation pressure with changes in temperature. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Hornback propane has a boiling point of -44 F -42 C at atmospheric pressure but methane natural gas has a boiling point of -260 F -162 C at atmospheric pressure. Further proof as if more was needed that. In the ABSENCE of other intermolecular force the higher the molecular mass the greater the boiling point.
These oils vary dramatically in appearance and physical properties like boiling point density odor and viscosity.
Look at the alkane series. 7837 C 1731 F Boiling point of methanol. The curve between the critical point and the triple point shows the propane boiling point with changes in pressure. Butane is perfect for any of your portable gas heaters and single-burner cooking appliances. This make propane more appropriate for exterior storage and use in cold climates. As with normal butane isobutane is a flammable.
 Source: thermopedia.com
Source: thermopedia.com
According to the textbook Organic Chemistry by Joseph M. They yield a variety of asphalt-like molecules. Propane will only cause bodily harm if liquid propane comes in contact with skin boiling point -44F. Propane boiling point is lower than butane so it will continue to vaporise from a liquid to a gas even in very cold weather down to -42C. Light crude is rich in low-boiling and paraffinic hydrocarbons.
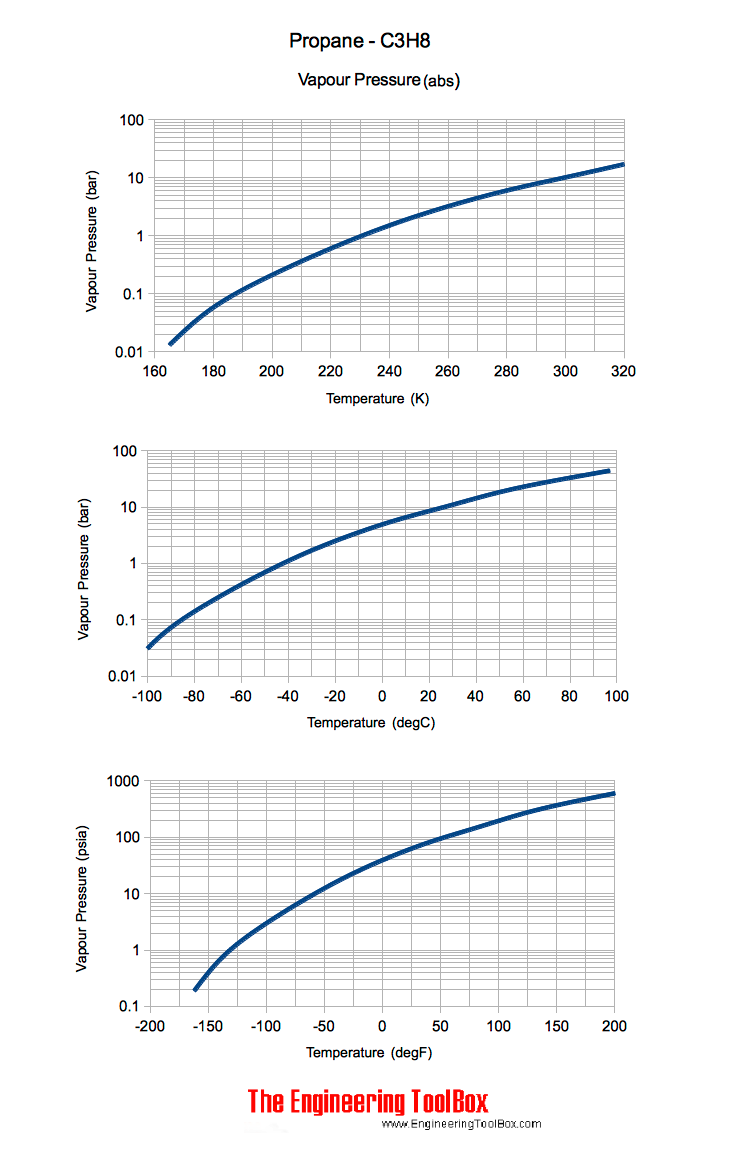 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. What are the Properties of Propane. As propane functions well in lower temperatures it is usually used for outdoor use. Typical liquids include propane ammonia carbon dioxide or nitrogen. I also got the matching Coleman 5-Ft.
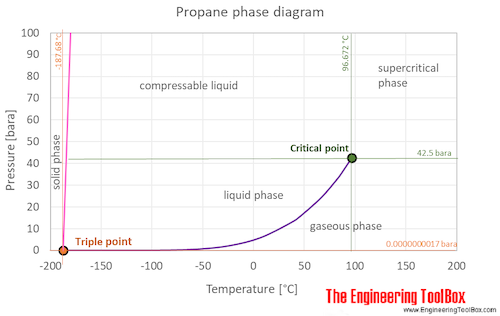 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. It quickly attached and the rest of this went together. As a final example I give you 2233-tetramethylbutane. C 7 H 16-91. When stored as propane liquid in a cylinder or tank it has 4x higher pressure than butane at the same temperatures.
 Source: youtube.com
Source: youtube.com
As a liquid it looks a lot like water. C 6 H 14-95. If this all seems rather ambiguous contradictory and imprecise well you have a point. Mixtures of the two are also common. Fuel Boiling Point o F Acetaldehyde.
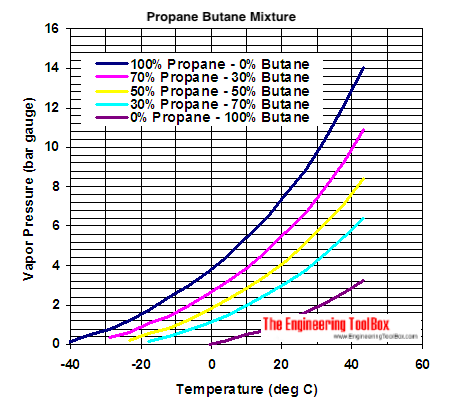 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
This means that propane will continue to vaporize and turn to gas in colder climates which is perfect for the cold winters we get here in Ontario and for outdoor use. The boiling point of water is typically considered to be 100 C or 212 F. Propane has a boiling point of -43 degrees which means that it can work in below-freezing temperatures unlike butane. Typical liquids include propane ammonia carbon dioxide or nitrogen. High elevation cooking generally takes longer since boiling point is a function of atmospheric pressure.
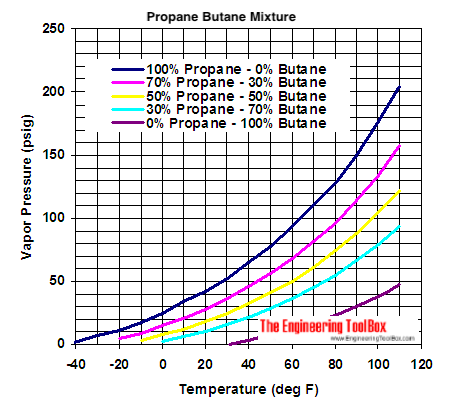 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Then again drinking water is harmless but who would attempt to chew on an ice cube that is 45 degrees below zero or take a 450 degree steam bath. 647 C 1485 F Boiling point of acetone. The longer the alkyl chain the greater the degree of dispersion force between molecules and thus the greater the boiling point. For making water potable As a. C 11 H 24-25.
 Source: schoolscience.co.uk
Source: schoolscience.co.uk
Propane has a boiling temperature of -42 C whilst butane has a higher boiling point at -2 C. Many of the co. It quickly attached and the rest of this went together. C 5 H 12-130. It is important to.
 Source: youtube.com
Source: youtube.com
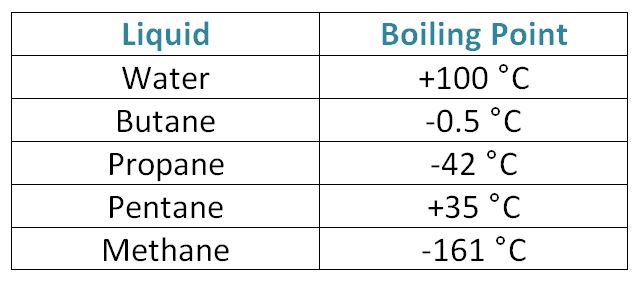
C 4 H 10-138-05. C 3 H 8-190-42. C 5 H 12-130. Highly branched vs. C 20 H 42.

The curve between the critical point and the triple point shows the propane boiling point with changes in pressure. In other words at 10 degrees below zero propane is well past its boiling point. It is important to. If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. 7837 C 1731 F Boiling point of nitrogen.
 Source: elgas.com.au
Source: elgas.com.au
Disposable cartridges for use in portable gas appliances. 12-November-2015 The Coleman Company Inc. -1958 C -3204 F Boiling point of liquid helium. Many of the co. 7837 C 1731 F Boiling point of methanol.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title propane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.