Potassium nitrate boiling point
Home » datasheet » Potassium nitrate boiling pointPotassium nitrate boiling point
Potassium Nitrate Boiling Point. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19. Salt becomes molten at a lower temperature than sand. Decomposes Solubility in water.

As an animal feed ingredient to satisfy the potassium requirements of farmed animals such as broiler breeder chickens. 400C 752F Melting Point. To separate the components a mixture of salt and sand is heated above 801C yet below 1710C. Potassium bromide is a solid. Usually this is not the most practical method of separation because both temperatures are very high. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography.
During a preparation of chlorine by addition of the concentrated acid Hydrochloric acid to solid potassium permanganate a sharp explosion occurred on one occasion.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. In condensed aerosol fire suppression although as the byproduct of potassium nitrate. Decomposes Solubility in water. As an ingredient in welding fluxes and in the flux coating on arc-welding rods. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. 1164 K Boiling point.
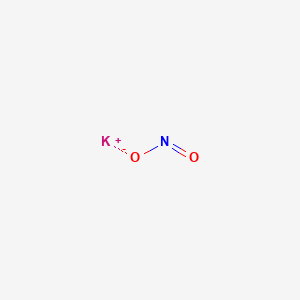
Also incompatible with boron and finely powdered metals chromium nitride aluminum titanium anitimony germanium zinc zirconium calcium disilicide metal. Decomposes Solubility in water. It was first isolated from potash the ashes of plants from which its name derives. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19. Of 5 potassium permanganate ammonium nitrate explosive caused an explosion 7 hrs.

Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. In condensed aerosol fire suppression although as the byproduct of potassium nitrate. 400C 752F Melting Point. Decomposes Solubility in water. Salt becomes molten at a lower temperature than sand.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. Addition of Potassium permanganate dimethylformamide to give a 20 solution led to an explosion after 5 min. Also incompatible with boron and finely powdered metals chromium nitride aluminum titanium anitimony germanium zinc zirconium calcium disilicide metal. 891 C 1636 F.

The melting point of salt is 1474F 801C while that of sand is 3110F 1710C. It was first isolated from potash the ashes of plants from which its name derives. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Potassium nitrate reacts vigorously when heated with sulfides of the alkaline earth group including barium sulfide and calcium sulfide. To separate the components a mixture of salt and sand is heated above 801C yet below 1710C.
 Source: chemicalbook.com
Source: chemicalbook.com
To separate the components a mixture of salt and sand is heated above 801C yet below 1710C. 1164 K Boiling point. Of 5 potassium permanganate ammonium nitrate explosive caused an explosion 7 hrs. In condensed aerosol fire suppression although as the byproduct of potassium nitrate. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19.
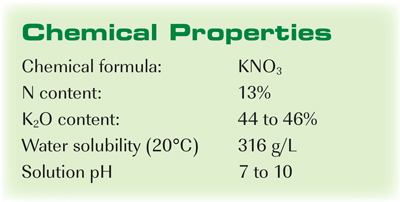 Source: cropnutrition.com
Source: cropnutrition.com
The molten salt may be poured off leaving the sand. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. During a preparation of chlorine by addition of the concentrated acid Hydrochloric acid to solid potassium permanganate a sharp explosion occurred on one occasion. Other uses for potassium bromide are in clear completion fluids in the petroleum industry as a pharmaceutical intermediate and in the manufacture of fibers. Potassium nitrate reacts vigorously when heated with sulfides of the alkaline earth group including barium sulfide and calcium sulfide.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting point of salt is 1474F 801C while that of sand is 3110F 1710C. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. Of 5 potassium permanganate ammonium nitrate explosive caused an explosion 7 hrs. During a preparation of chlorine by addition of the concentrated acid Hydrochloric acid to solid potassium permanganate a sharp explosion occurred on one occasion. Salt becomes molten at a lower temperature than sand.

As an ingredient in welding fluxes and in the flux coating on arc-welding rods. 891 C 1636 F. Of 5 potassium permanganate ammonium nitrate explosive caused an explosion 7 hrs. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. Potassium nitrate reacts vigorously when heated with sulfides of the alkaline earth group including barium sulfide and calcium sulfide.
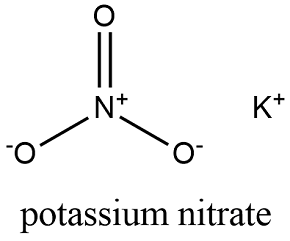 Source: softschools.com
Source: softschools.com
Other uses for potassium bromide are in clear completion fluids in the petroleum industry as a pharmaceutical intermediate and in the manufacture of fibers. In condensed aerosol fire suppression although as the byproduct of potassium nitrate. As an ingredient in welding fluxes and in the flux coating on arc-welding rods. Other uses for potassium bromide are in clear completion fluids in the petroleum industry as a pharmaceutical intermediate and in the manufacture of fibers. The melting point of salt is 1474F 801C while that of sand is 3110F 1710C.
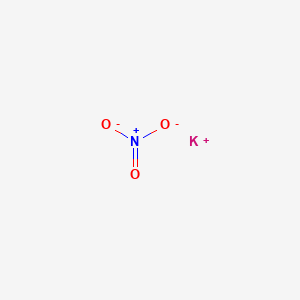
To separate the components a mixture of salt and sand is heated above 801C yet below 1710C. As an ingredient in welding fluxes and in the flux coating on arc-welding rods. 400C 752F Melting Point. Potassium bromide may be utilized for the production of photographic-grade silver bromide in process engraving and lithography. To separate the components a mixture of salt and sand is heated above 801C yet below 1710C.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title potassium nitrate boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.