Potassium hydroxide melting point
Home » datasheet » Potassium hydroxide melting pointPotassium hydroxide melting point
Potassium Hydroxide Melting Point. POTASSIUM HYDROXIDE SOLUTION is a strong base dissolved in water. 6328 C 14590 F boiling point. The resulting solution is basic because of the dissolved hydroxide. NH 4 NO 3 aq KOH aq NH 3 g KNO 3 aq H 2 O l An alternative way of producing potassium nitrate without a by-product of ammonia is to combine ammonium nitrate found in instant ice packs 16 and potassium chloride easily obtained as a sodium-free salt substitute.
 Physical Properties Of Koh Download Table From researchgate.net
Physical Properties Of Koh Download Table From researchgate.net
Potassium hydroxide Structure KOH. Properties occurrence and uses. Worldwide production in 1998 was around 45 million tonnes. It reacts with water violently and gives hydrogen which can actually catch fire and may explode. 1 hPa 719 C Solubility. The name is derived from the english word potash.
It constitutes 26 percent of Earths mass.
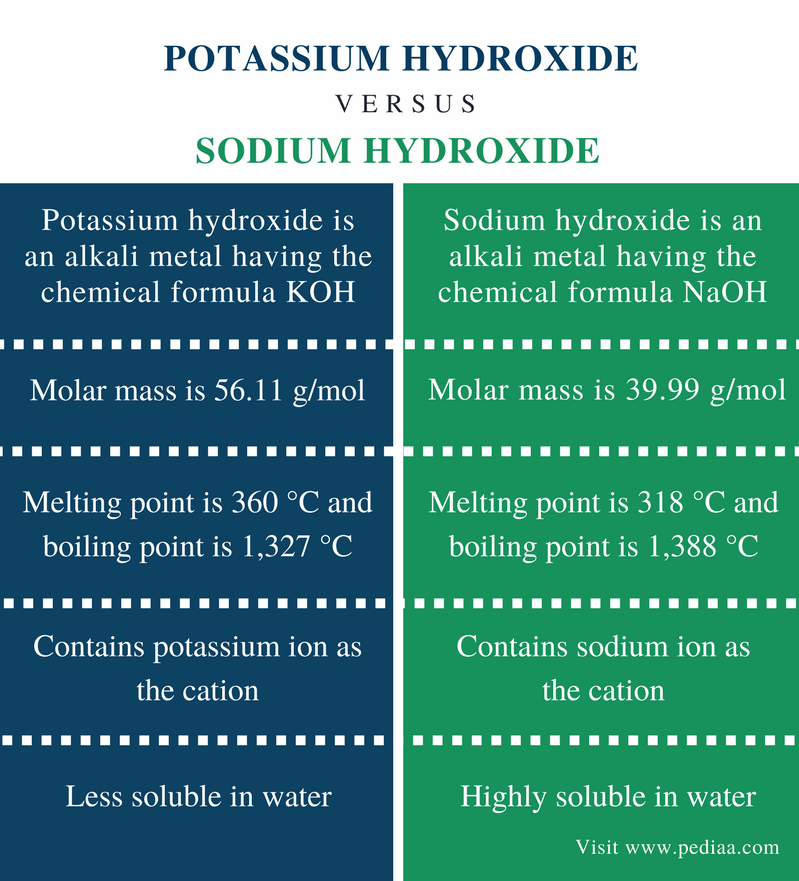
It is soft and shiny metal which has a melting point 63 degrees and the boiling point as 770 degrees. Potassium hydroxide also known as lye is an inorganic compound with the chemical formula KOHAlso commonly referred to as caustic potash it is a potent base that is marketed in several forms including pellets flakes and powdersIt is used in various chemical industrial and manufacturing applications. Potassium has two oxidation states 1 and -1rarely. The element whose atomic number is 19 and atomic weight 39098u has a melting point of 6328C and a boiling point of 760C. Potassium hydroxide solution is more conductive when compared to NaOH and therefore used as an electrolyte in some alkaline batteries. It has a green vapour and lavender coloured flame.
 Source: fishersci.pt
Source: fishersci.pt
Ignited a polyethylene container liner when mixed with potassium persulfate by release of heat and oxygen MCA Case History 1155. Physical and chemical properties Physical State Solid Appearance Light yellow Odor Odorless Odor Threshold No information available pH 135 01M Melting PointRange 360 C 680 F Boiling PointRange 1320 C 2408 F Flash Point Not applicable Evaporation Rate Not applicable. Potassium has two oxidation states 1 and -1rarely. 135 56 gl H₂O 25 C Vapor pressure. In exceptional circumstances membership may be granted to an applicant who does not meet the criteria for research productivity but for whom there is evidence of particular academic distinction and an especially good fit with the Institutes research focus areas.
 Source: pediaa.com
Source: pediaa.com
It is used in chip. Physical and chemical properties Physical State Solid Appearance Light yellow Odor Odorless Odor Threshold No information available pH 135 01M Melting PointRange 360 C 680 F Boiling PointRange 1320 C 2408 F Flash Point Not applicable Evaporation Rate Not applicable. Stable at room temperature in closed containers under normal. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Potassium has two oxidation states 1 and -1rarely.
Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas. Electronic shell Ar 4s 1. Along with sodium hydroxide NaOH KOH is a prototypical strong baseIt has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acidsAn estimated 700000 to 800000 tonnes were produced in 2005. It is used in the thickening of food. 1390 deg C 760 mm Hg.
 Source: researchgate.net
Source: researchgate.net
Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash. Stable at room temperature in closed containers under normal. It is ranked as the 7th most. Uses of Potassium Hydroxide. Potassium nitrate can be made by combining ammonium nitrate and potassium hydroxide.
 Source: researchgate.net
Source: researchgate.net
Attacks aluminum and zinc to generate flammable hydrogen gas. Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash. Stable at room temperature in closed containers under normal. The resulting solution is basic because of the dissolved hydroxide. Sodium hydroxide is also the most common base used in chemical laboratories.

Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. It is soft and shiny metal which has a melting point 63 degrees and the boiling point as 770 degrees. Electronic shell Ar 4s 1. 1390 deg C 760 mm Hg. Sodium hydroxide is also the most common base used in chemical laboratories.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Readily absorbs carbon dioxide and moisture from the. LD50 Rat 333 mgkg. Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash. 2-8-8-1 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Potassium carbonate K2CO3 is a white salt soluble in water insoluble in ethanol which forms a strongly alkaline solutionIt can be made as the product of potassium hydroxides absorbent reaction with carbon dioxideIt presents a large capacity to absorb moisture.
 Source: yumpu.com
Source: yumpu.com
2-8-8-1 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Reacts exothermically with all acids. Potassium metal reacts very rapidly with water to form a colourless solution of potassium hydroxide KOH and hydrogen gas H 2. It constitutes 26 percent of Earths mass. Sir Davy in 1808.
 Source: studylib.net
Source: studylib.net
Soluble in water Specific GravityDensity204 Molecular FormulaKOH Molecular Weight561 Section 10 - Stability and Reactivity Chemical Stability. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. It is soft and shiny metal which has a melting point 63 degrees and the boiling point as 770 degrees. Potassium metal can float on water. Worldwide production in 1998 was around 45 million tonnes.

Ignited a polyethylene container liner when mixed with potassium persulfate by release of heat and oxygen MCA Case History 1155. It constitutes 26 percent of Earths mass. 1 hPa 719 C Solubility. Electronic shell Ar 4s 1. Worldwide production in 1998 was around 45 million tonnes.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium hydroxide melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.